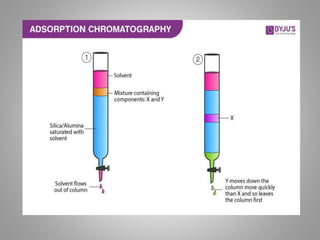
This document summarizes adsorption chromatography, a method used to separate mixtures. It was developed by American scientist D.T. Day and later botanist M.S. Tswett used adsorption columns to investigate plant pigments. The principle is that components are separated based on how strongly they adsorb to the column material, with the most strongly adsorbed at the top and least at the bottom. Common adsorbents discussed include silica gel, alumina, fuller's earth, charcoal, and polystyrene beads. The column is prepared either through dry packing or wet packing of the adsorbent material. Adsorption chromatography is used to separate mixtures such as polycyclic aromatic