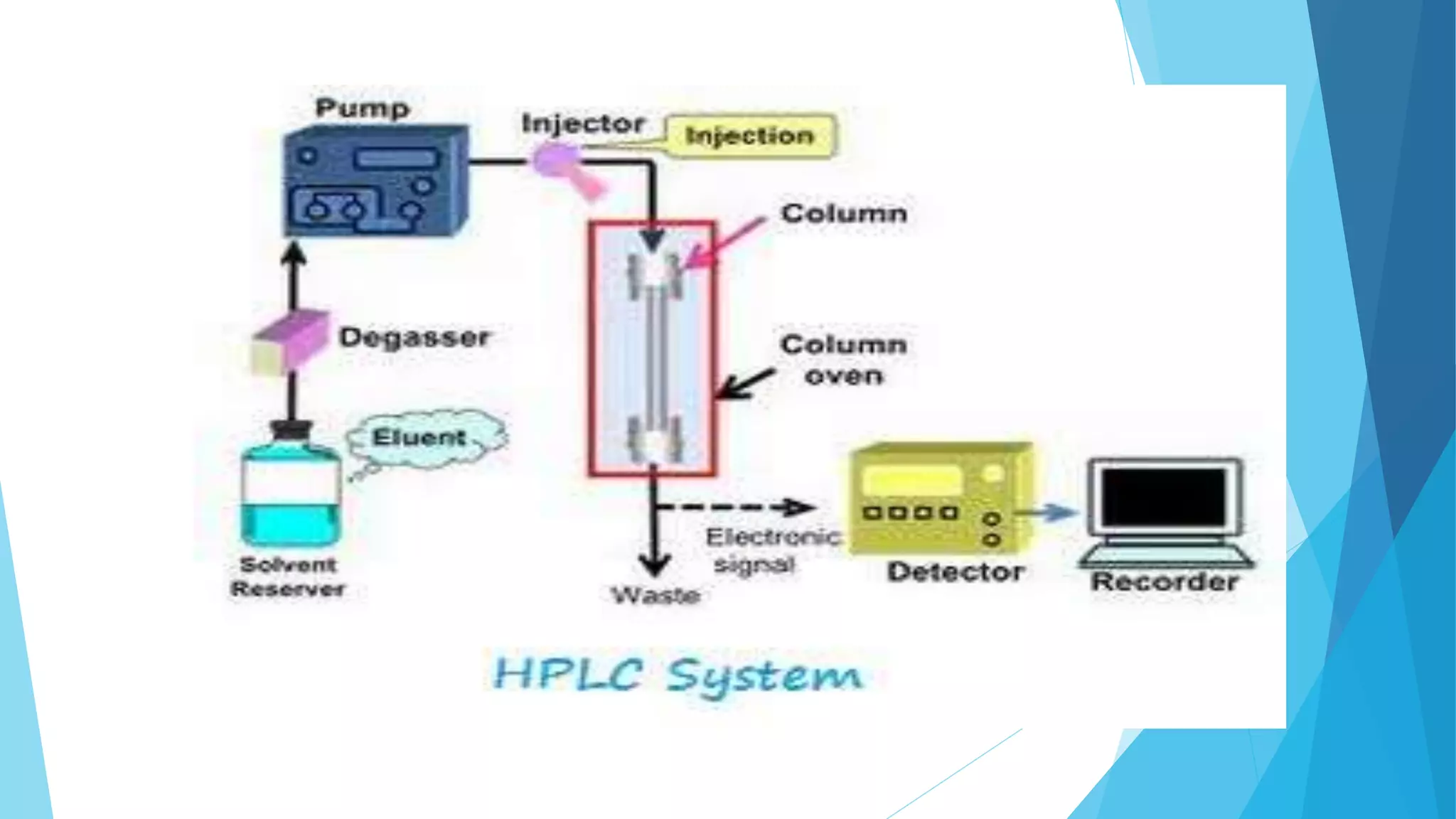
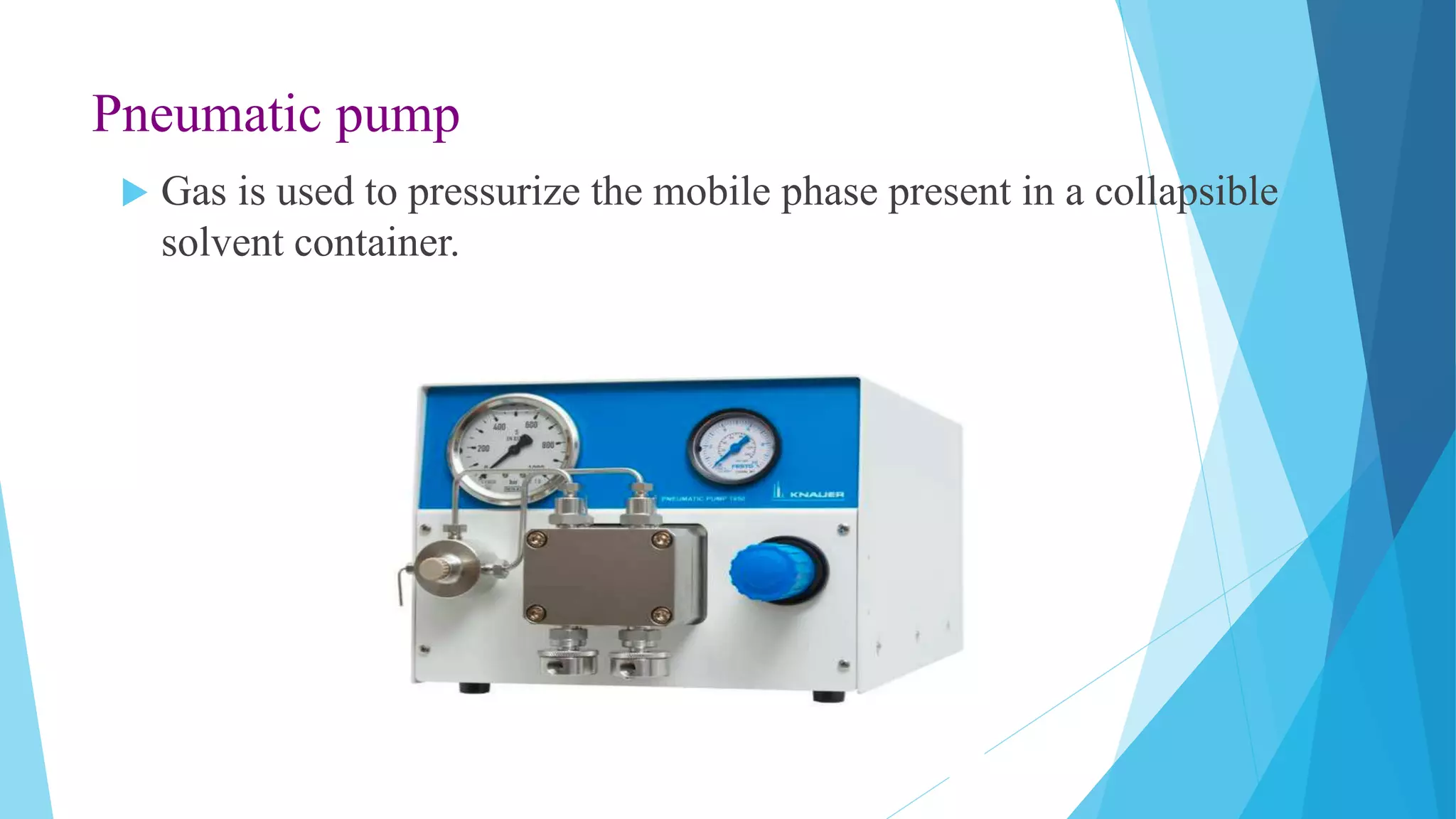
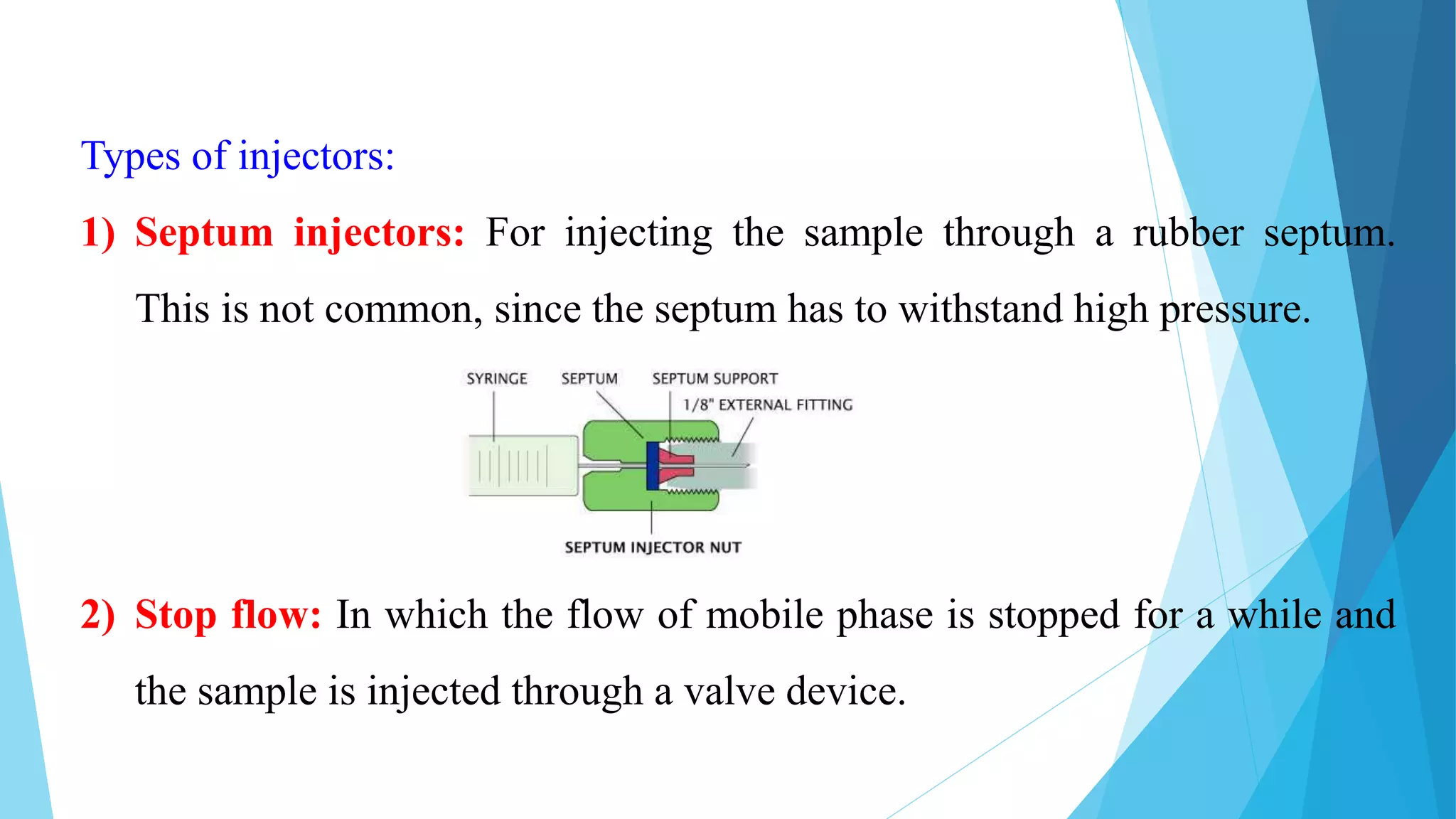
HPLC is a chromatographic technique used to separate components of a mixture. The key components of an HPLC instrument are the pump, injector, column, and detectors. The pump forces the mobile phase through the column while the injector introduces the sample. Various detectors can then analyze the separated components as they elute from the column. HPLC is widely used in fields like biochemistry and pharmaceutical analysis for applications such as quantifying drug purity and identifying unknown compounds.