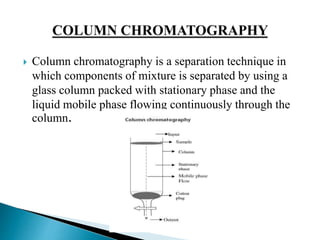
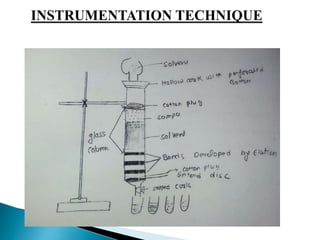
This document provides an overview of column chromatography, including its history, definition, principle, types, experimental technique, requirements, applications, advantages, and disadvantages. Column chromatography was developed in 1901 by Russian botanist Mikhail Tsvet as a method to separate plant pigments by passing an organic solution through an adsorptive material in a glass column, resulting in discrete colored bands. It involves using a column packed with a stationary phase and flowing a liquid mobile phase through to separate components of a mixture based on differential adsorption between the phases.