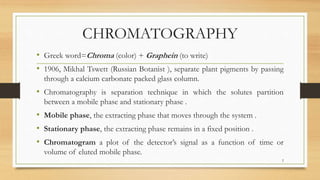
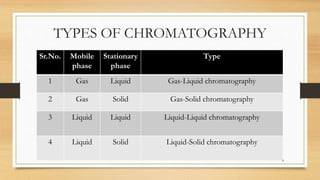
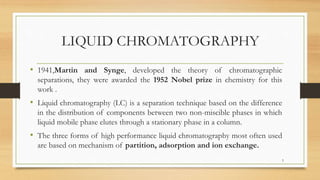
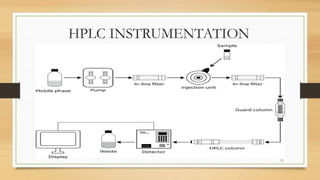
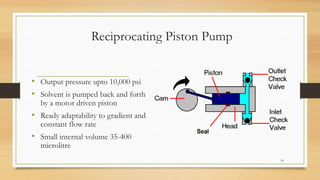
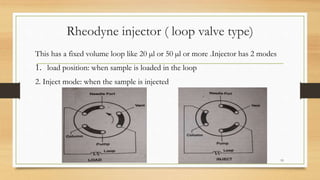
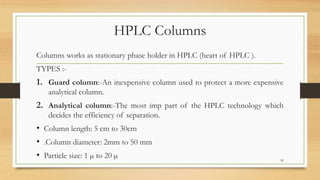
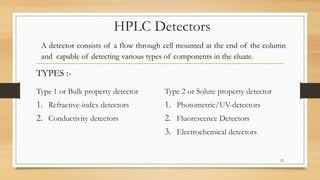
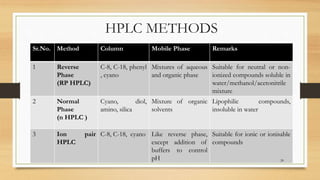
The document discusses High Performance Liquid Chromatography (HPLC), a separation technique developed by Huber in 1969, which involves passing a sample through a column under high pressure, enabling the separation of mixtures based on their affinities for stationary and mobile phases. It outlines various types of chromatography, the components of HPLC apparatus, and the methods and applications of HPLC, including its use in quantitative and qualitative analysis. Key features include high resolving power, speed of separation, and automation of the analytical process.