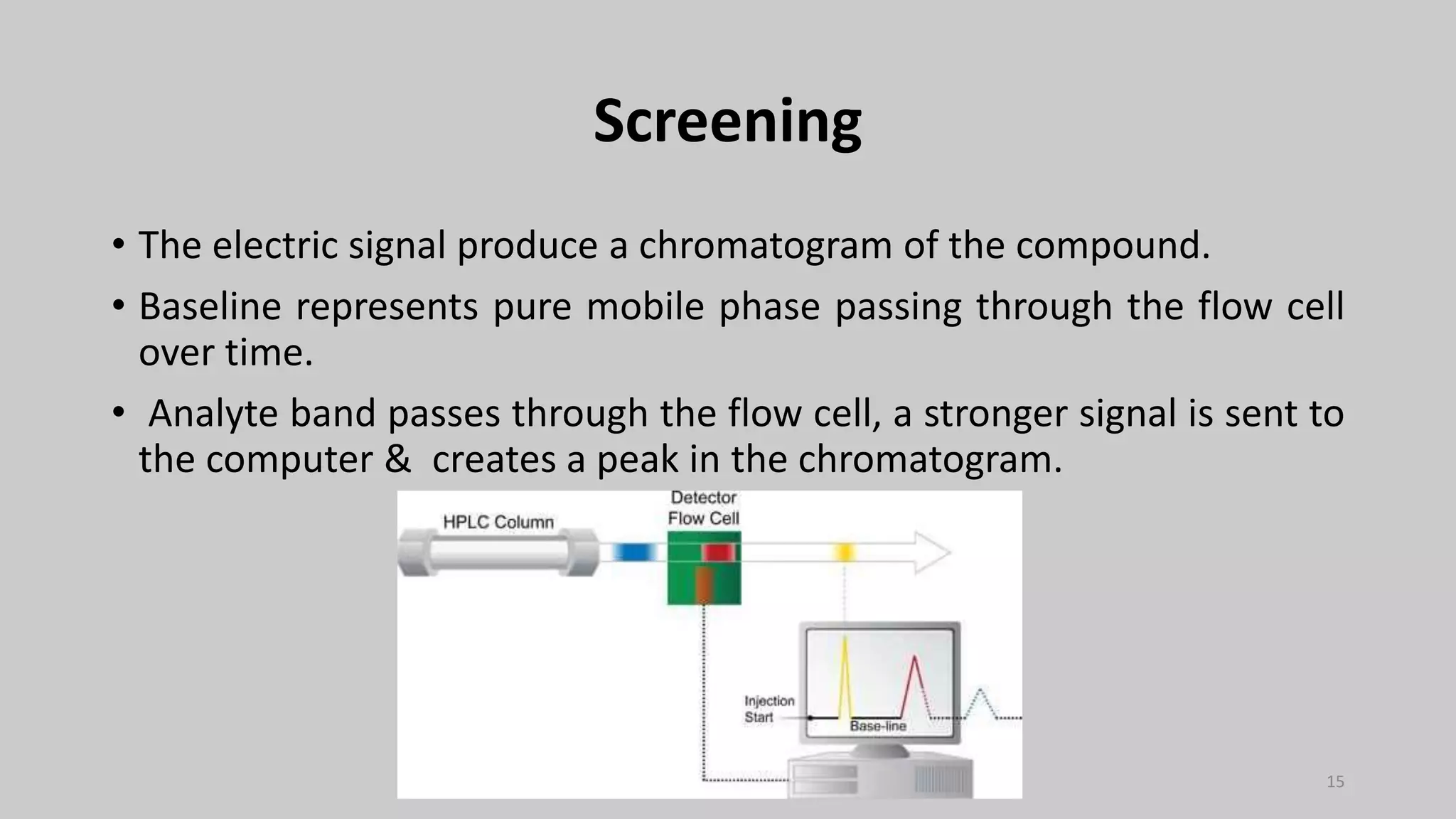
The document provides an overview of High-Performance Liquid Chromatography (HPLC), detailing its definition, historical development, principles, types, components, and applications. HPLC is a key analytical technique used for separating, identifying, and quantifying organic compounds in various fields, including pharmaceuticals and environmental science. It highlights the advantages and disadvantages of HPLC, comparisons with other methods like gas chromatography, and essential factors influencing its performance.