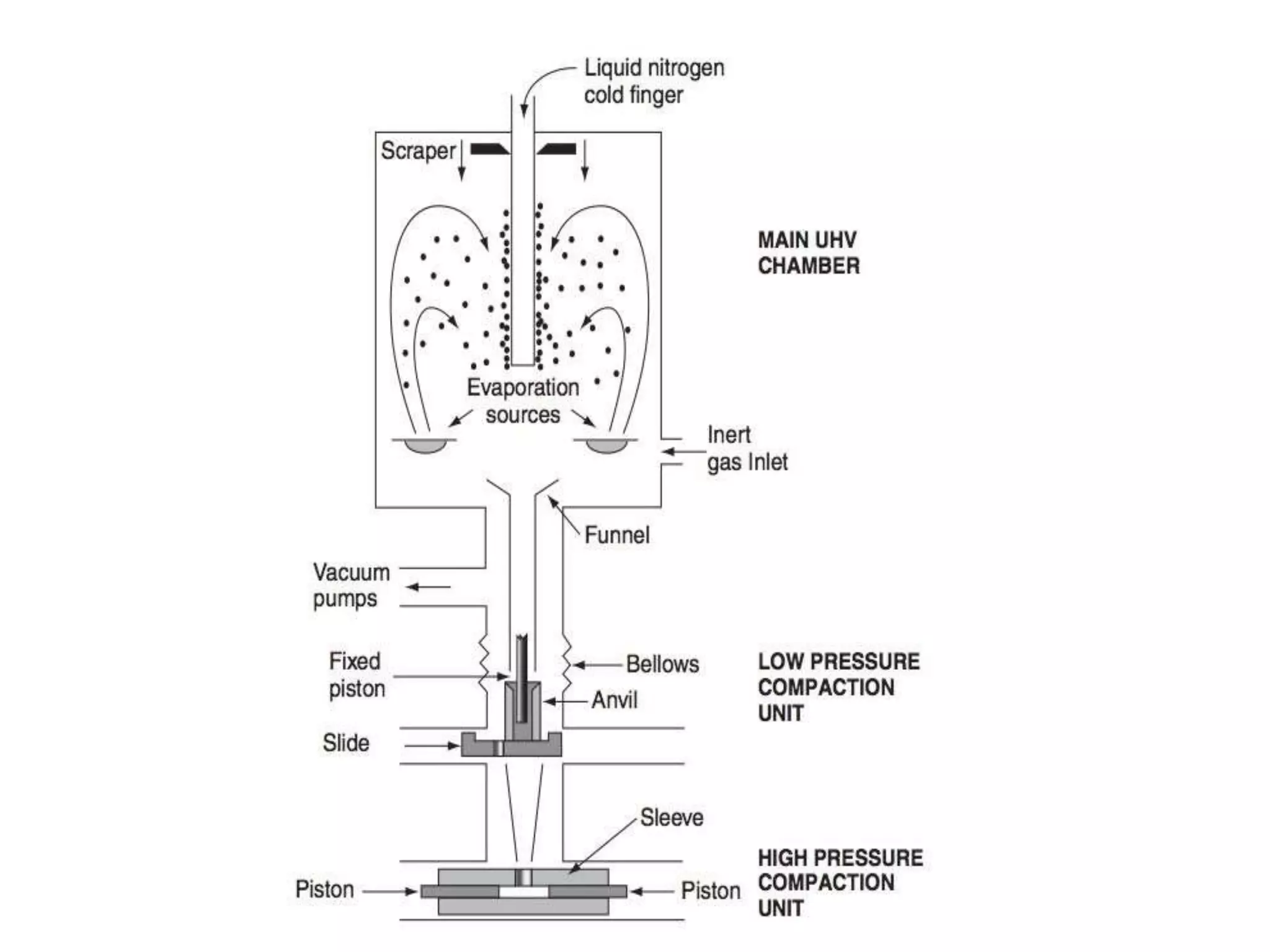
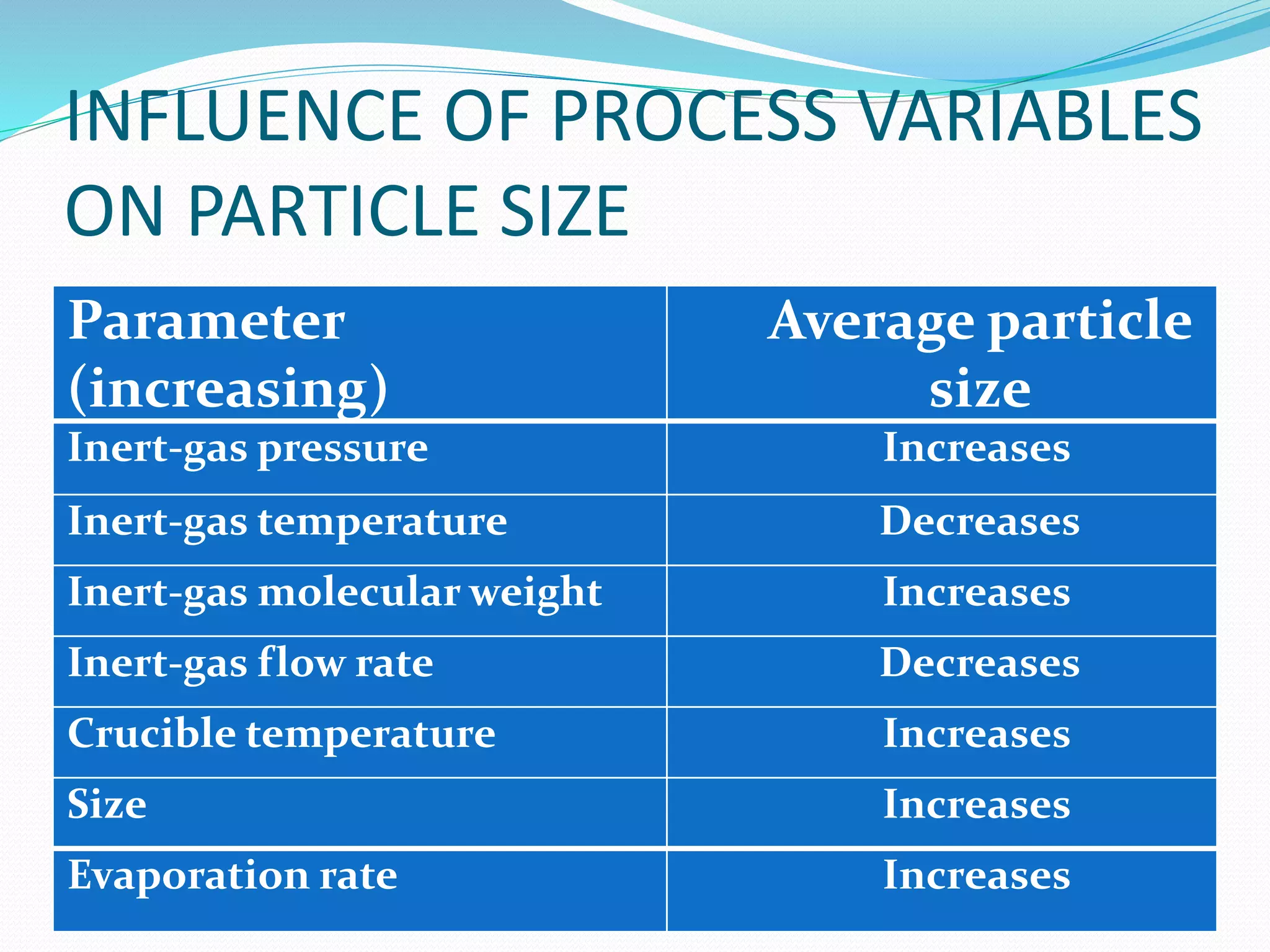
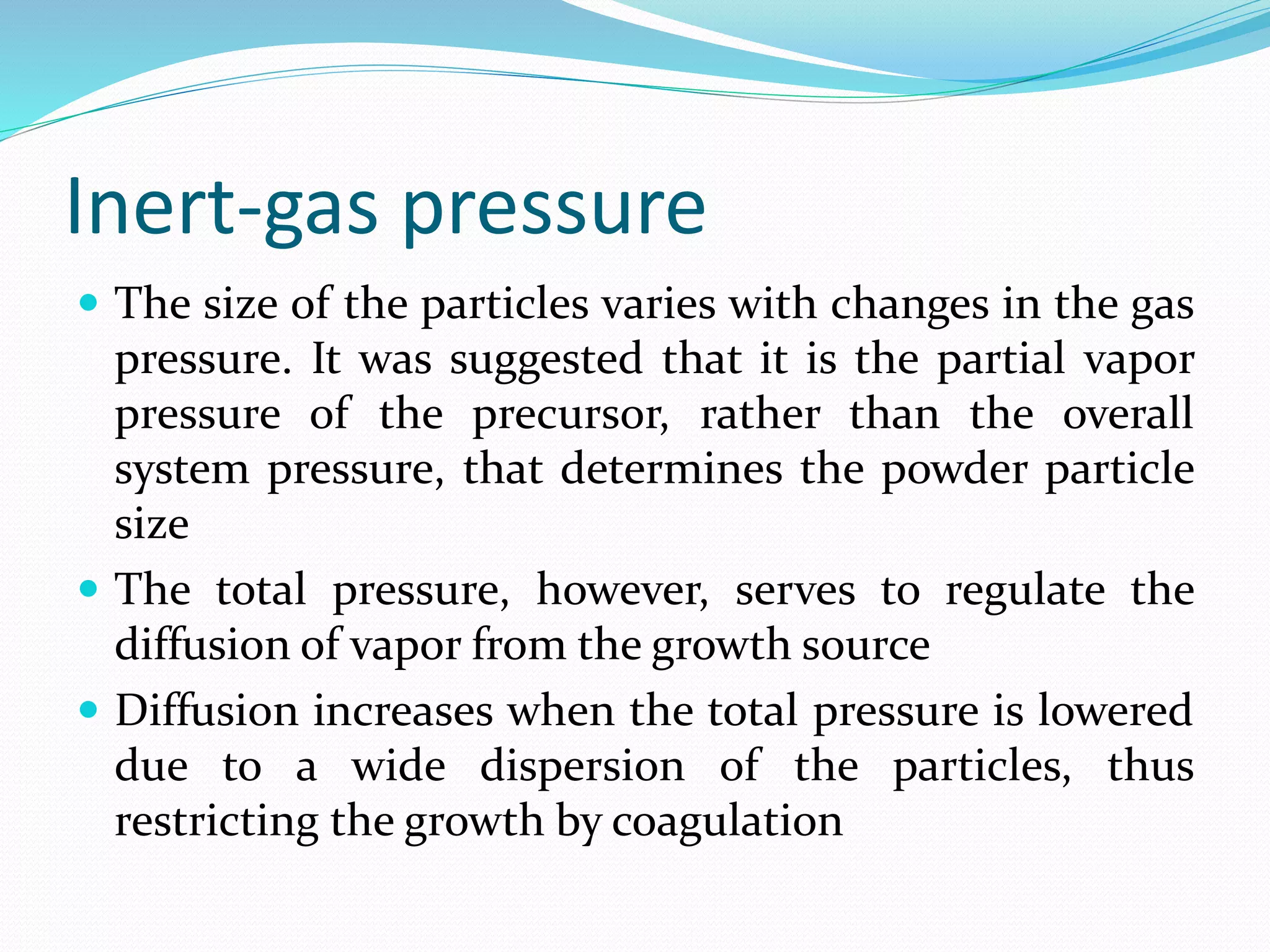
The document summarizes the inert gas condensation method for preparing nanoparticles. It involves evaporating a material and then rapidly condensing it using an inert gas to produce nanoparticles with controlled sizes in the range of 10-9 m. Process parameters like inert gas pressure, temperature, flow rate and evaporation rate can be adjusted to control the average particle size. This technique is used to produce a wide range of metallic, ceramic, and composite nanoparticles and offers advantages of size control and material flexibility, though high vacuum and agglomeration issues exist.