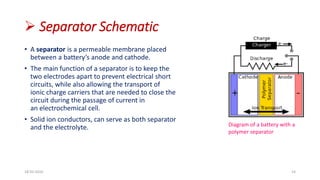
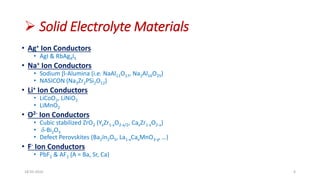
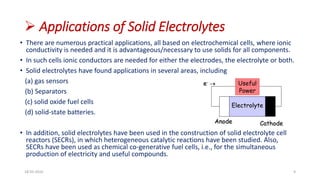
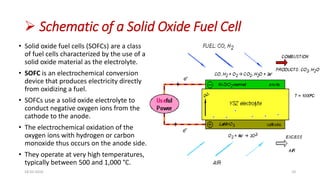
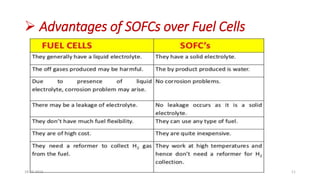
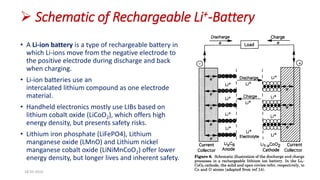
This document discusses the electrical properties of solid inorganic materials. It begins by defining solid electrolytes as crystalline solids that conduct electricity via the movement of ions. Some key solid electrolyte materials discussed include silver iodide (AgI), sodium beta-alumina, and lithium cobalt oxide (LiCoO2). Applications of solid electrolytes mentioned include use in solid oxide fuel cells, lithium-ion batteries, oxygen gas sensors, and as separators in electrochemical cells.






![ O2 Gas Sensor
• The partial pressure of oxygen in the
sample gas, PO2(sample), can be
determined from the measured potential,
V, via the Nernst equation.
V = (RT/4F) ln[(PO2(ref))/(PO2(sample))]
• Because of the low ionic conductivity at
low temperatures, the sensor is only useful
above 650 ºC.
• High concentration of anion vacancies is
necessary for O2- hopping to occur.
• Its two electrodes provide an output
voltage corresponding to the quantity of
oxygen in the exhaust relative to that in the
atmosphere.
18-03-2016 13](https://image.slidesharecdn.com/electricalpropertiesofsolids-170615154704/85/Electrical-properties-of-solids-13-320.jpg)