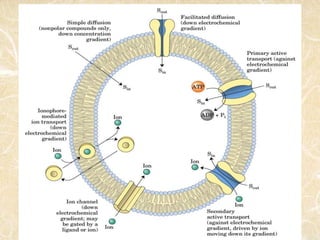
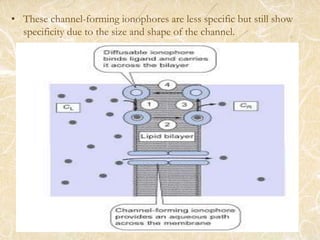
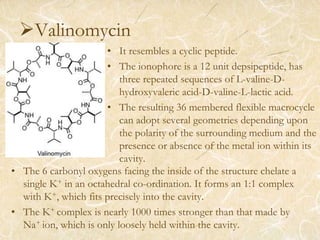
Ion transport across membranes can occur actively through ion pumps or passively through channels or carriers. Passive transport of metal ions can be carrier-mediated by ionophores, which are ligands that encapsulate metal ions and have organic groups to transport them across membranes. Examples of ionophores include valinomycin and nonactin, which selectively transport potassium ions and sodium ions respectively by binding them. Ionophores disrupt ion balance in cells and can act as antibiotics, though they cannot distinguish between microbial and host cells.