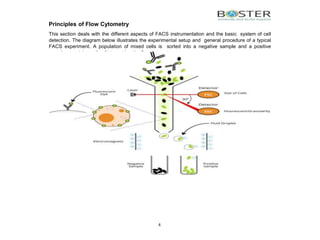
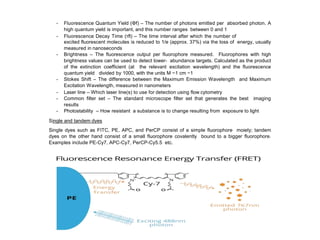
The document provides an in-depth overview of flow cytometry, detailing its principles, instrumentation, and the processes involved in cell detection and sorting. It explains the components of a flow cytometer, including the fluidics, optics, and electronics systems, and elaborates on how laser-induced fluorescence is measured to analyze cell populations. Additionally, the document discusses the properties of various fluorophores and the importance of proper filter settings, as well as considerations for spectral overlap and compensation during multi-color detection.