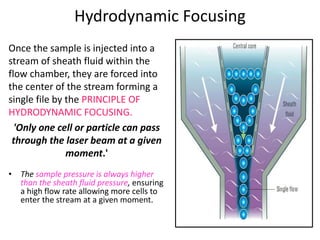
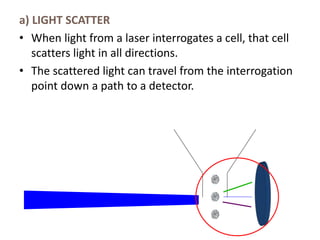
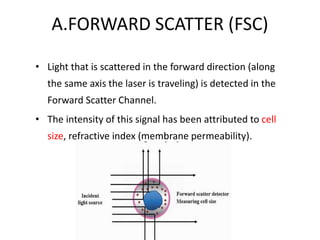
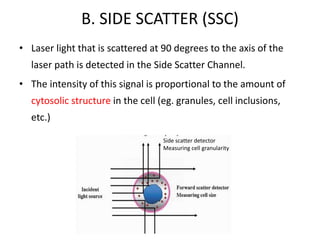
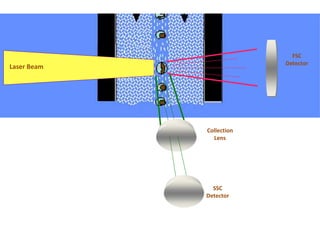
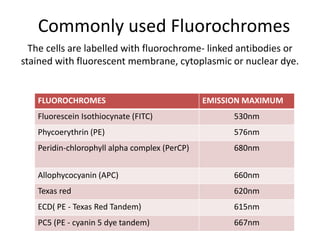
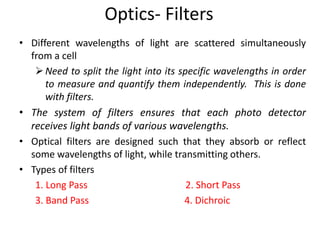
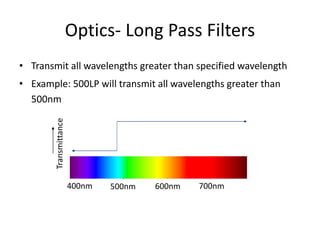
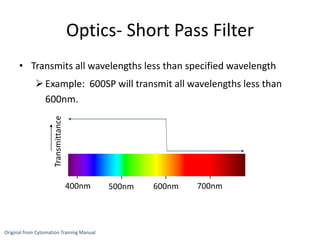
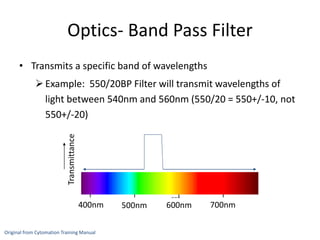
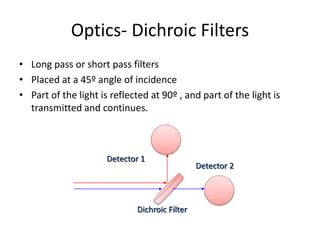
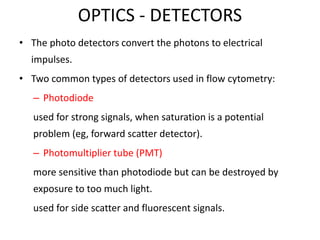
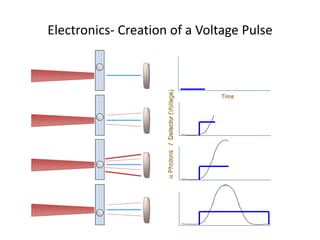
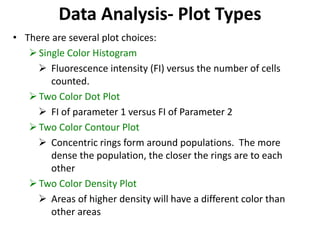
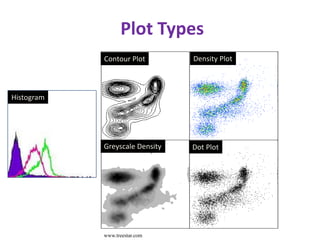
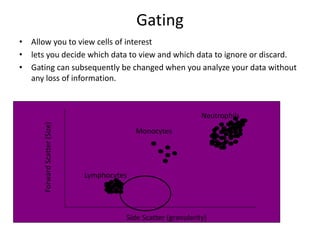
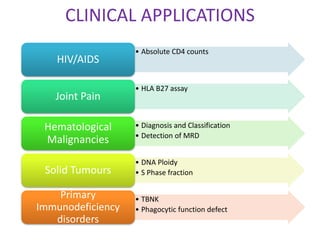
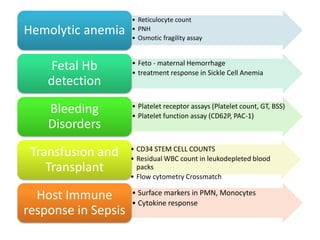
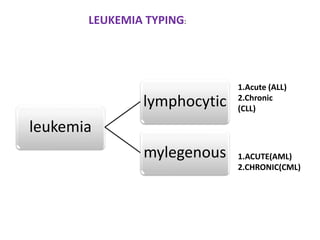
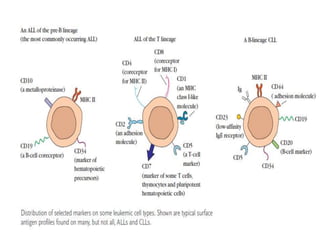
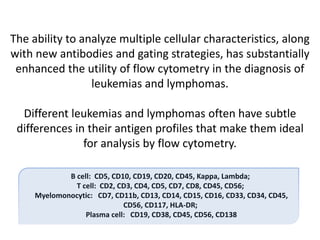
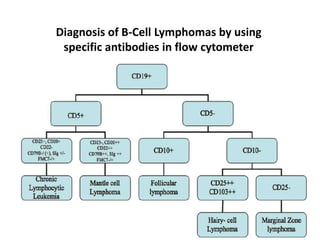
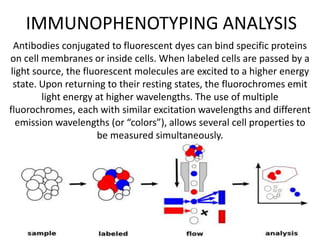
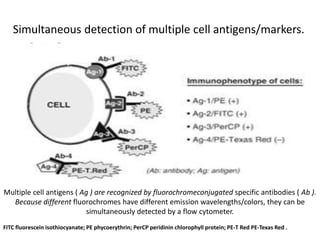
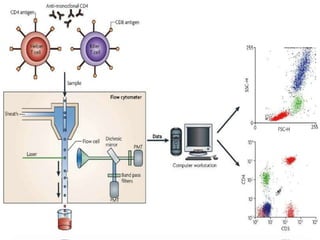
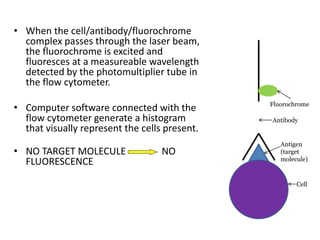
This document discusses flow cytometry, which measures properties of cells as they flow through a fluid stream. It describes the principles and components of a flow cytometer, including the flow system that orders cells into a single-file stream, the optical system that illuminates cells and detects light scattering/fluorescence, and the electronic system that converts signals to digital data. The document outlines how flow cytometry is used to analyze physical/antigen characteristics of cells and identifies different cell types. It provides examples of clinical applications like leukemia diagnosis and CD4 counting in HIV/AIDS.