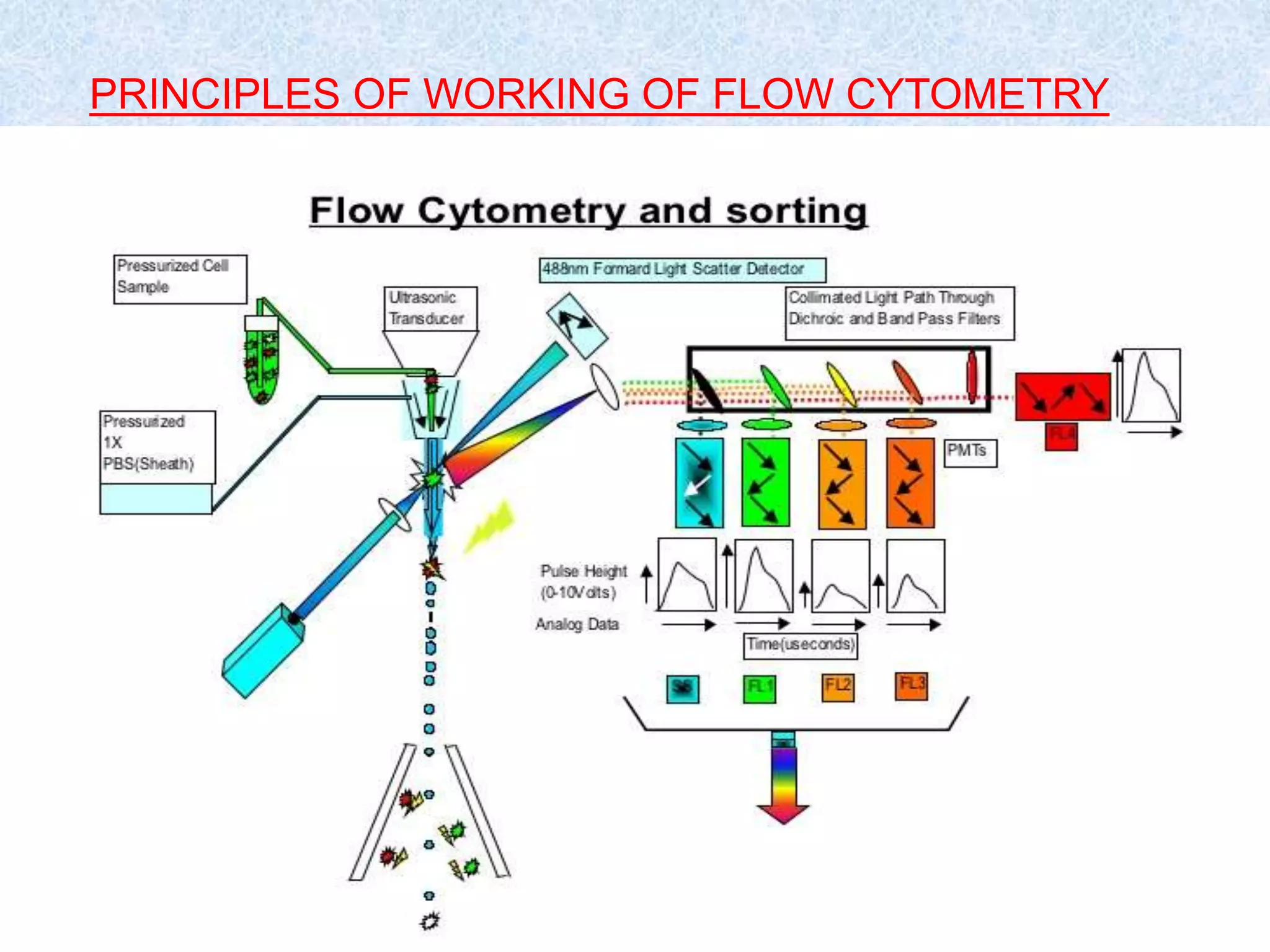
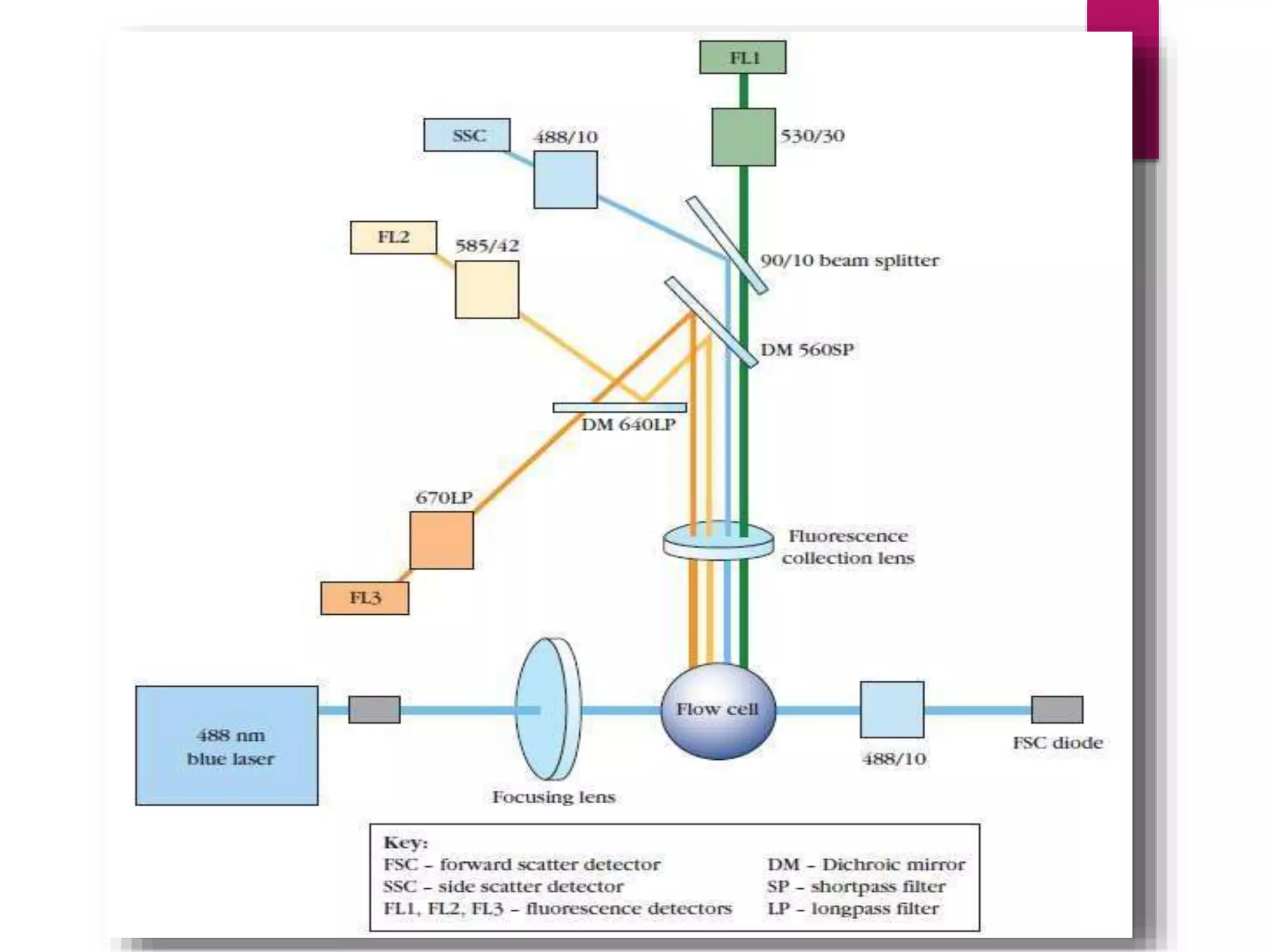
Flow cytometry is a laser-based technique used for analyzing physical and chemical properties of cells in a fluid stream, essential for applications such as cell counting, sorting, and determining cell cycle phases. The flow cytometer consists of fluidics, optics, and electronics systems that work together to transport and analyze particles, using fluorescent dyes to provide detailed cellular information. Its applications extend across fields including clinical diagnostics for cancers, immunophenotyping, and ecological studies of microorganisms.