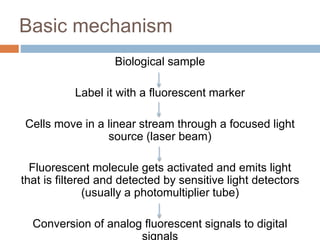
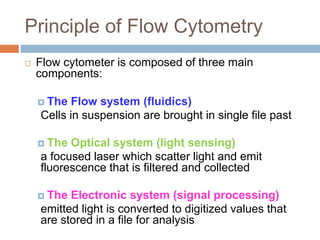
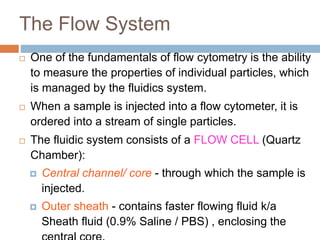
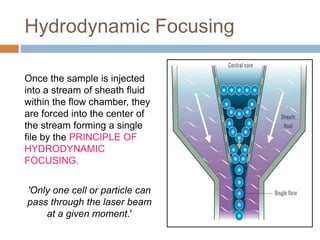
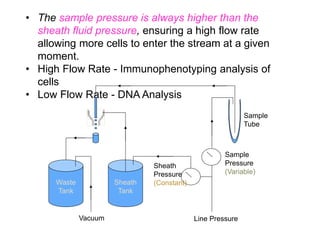
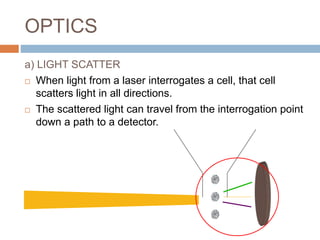
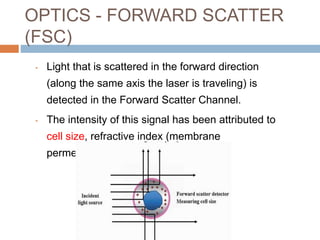
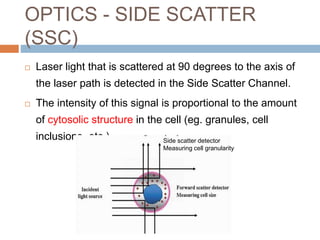
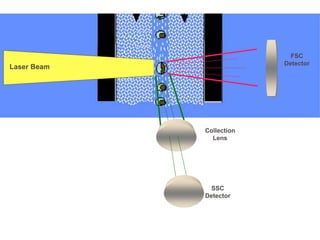
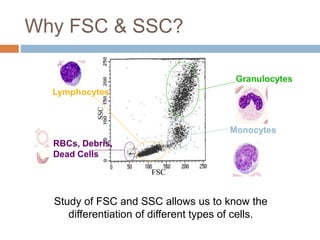
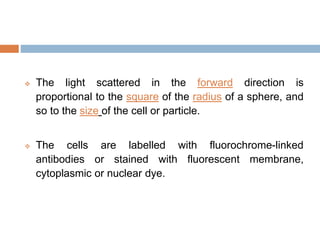
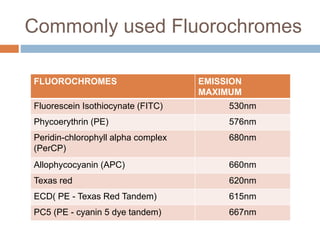
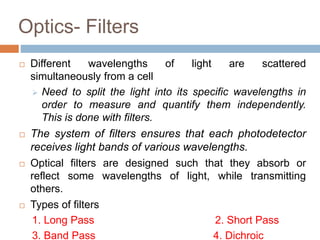
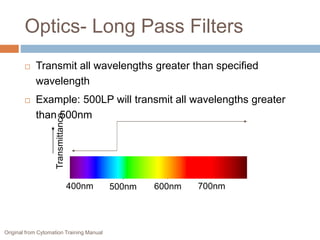
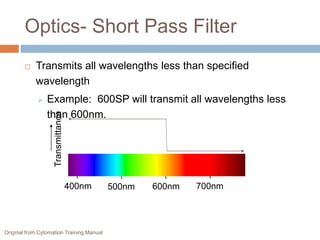
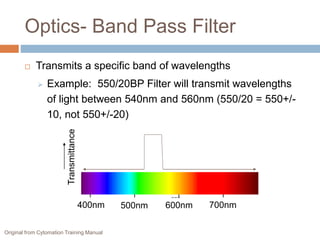
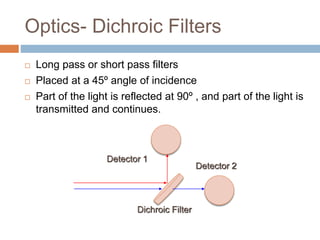
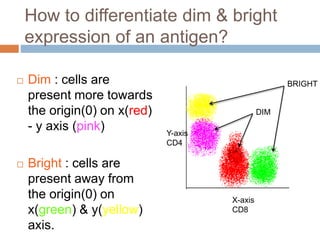
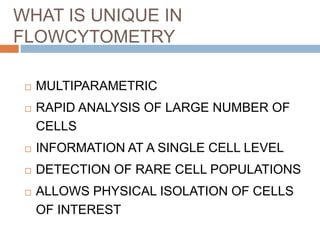
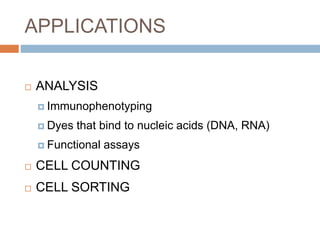
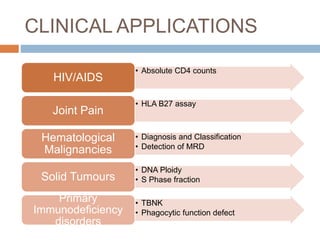
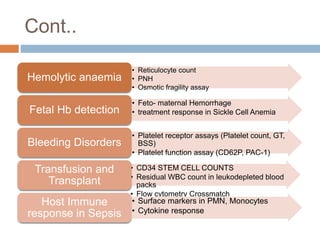
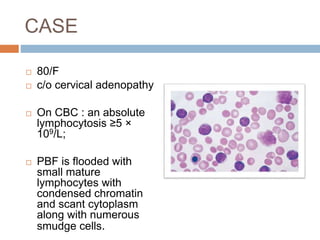
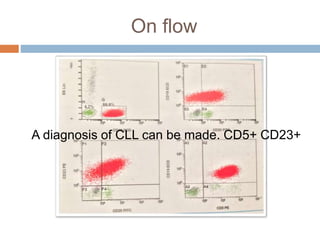
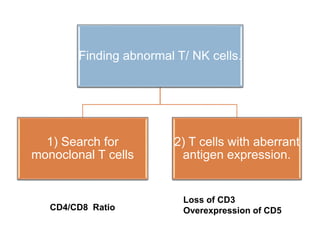
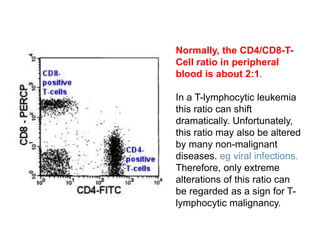
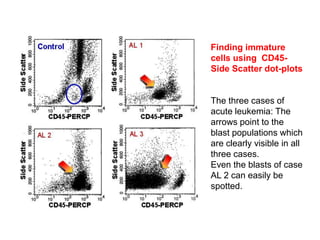
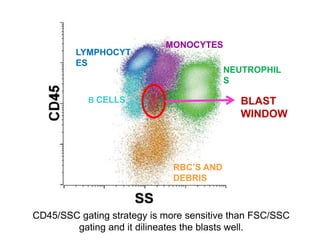
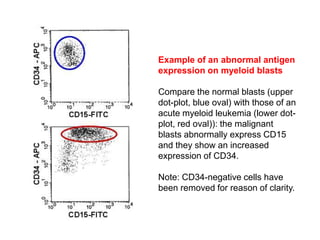
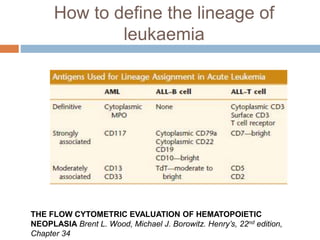
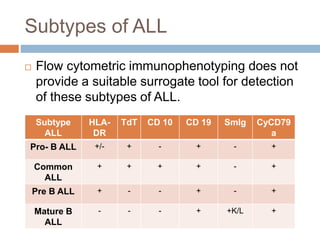
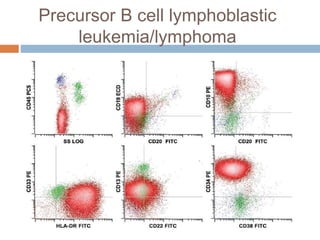
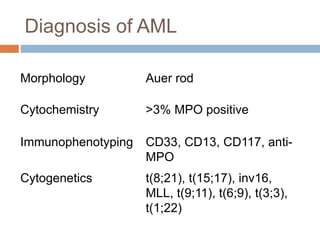
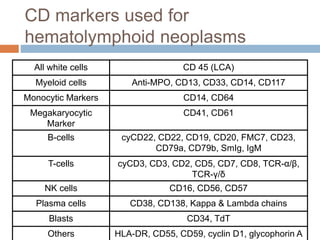
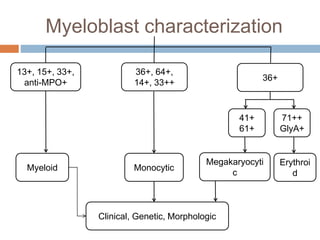
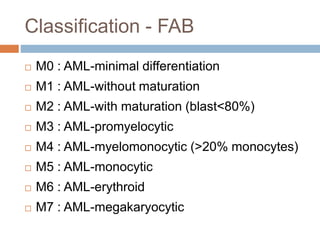
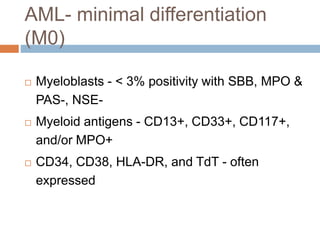
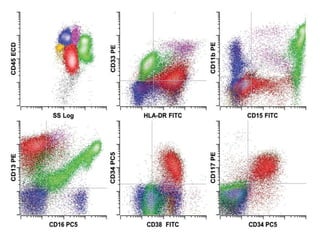
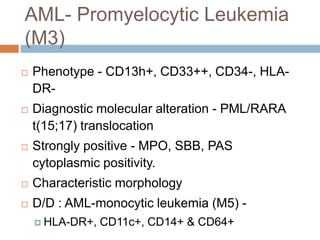
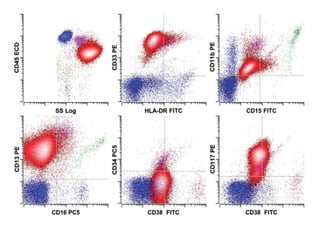
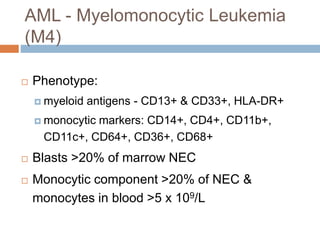
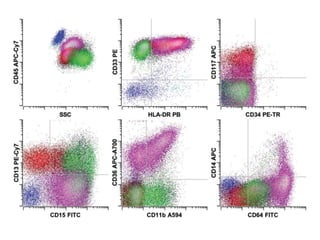
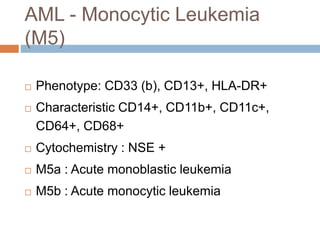
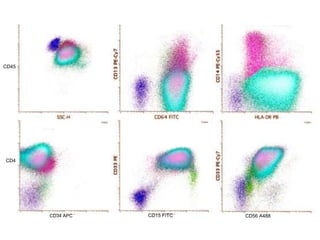
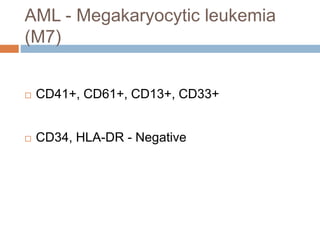
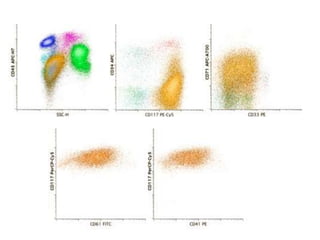
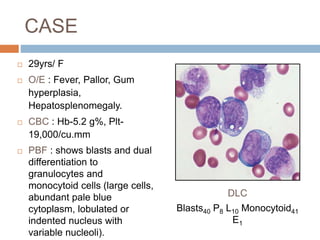
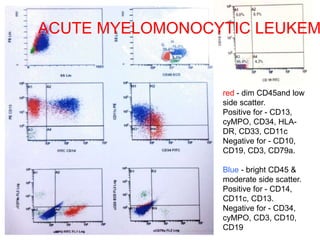
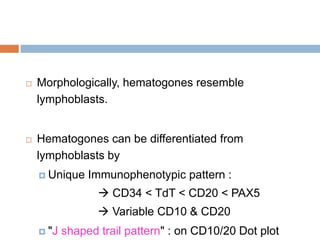
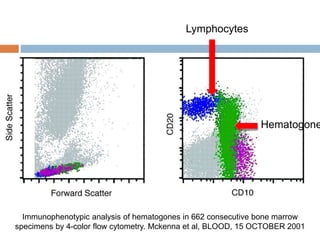
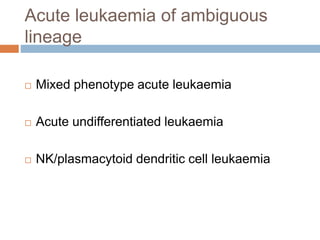
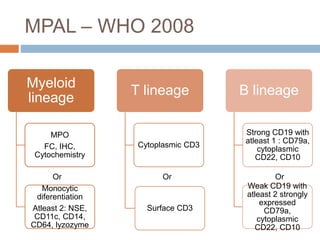
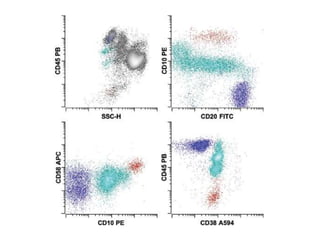
Flow cytometry allows for the quantitative and qualitative analysis of cell properties as cells flow in a fluid stream through a laser. Cells are labeled with fluorescent markers and pass through the laser one by one. Light scattering and fluorescence emission are converted to digital signals which can be analyzed as histograms or dot plots. Properties like cell size, granularity, and marker expression are measured. Gating allows analysis of specific cell populations. Flow cytometry has many applications including immunophenotyping, cell cycle analysis, and functional assays.