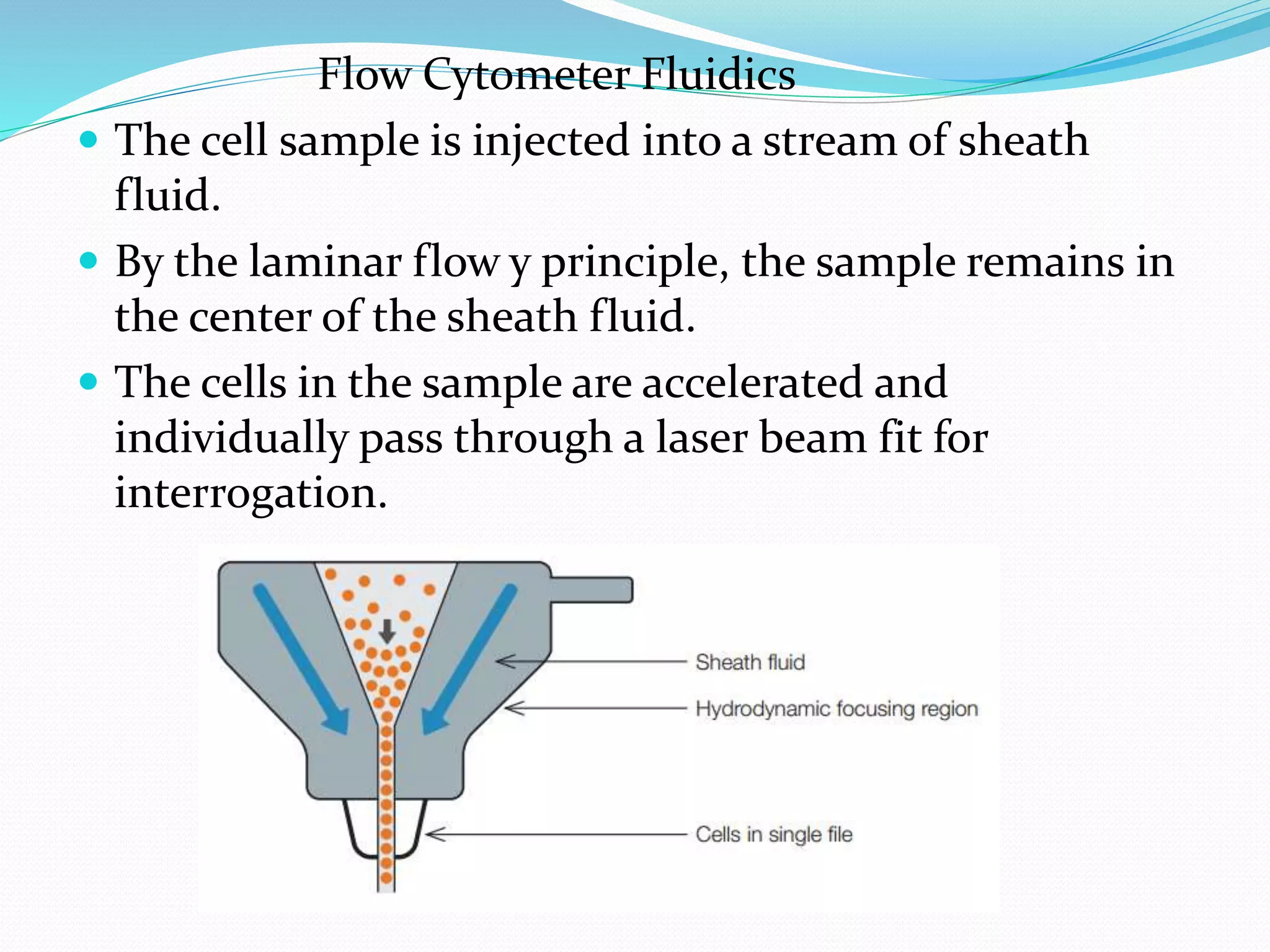
The document provides an overview of flow cytometry, a technique used to analyze and sort microscopic objects in a fluid based on their optical properties. It details the components of a flow cytometer, such as fluidics, optics, and electronics, as well as the roles of fluorochromes in immunophenotyping and data analysis. Additionally, it outlines both clinical and research applications, including the monitoring of CD4+ T cell counts in HIV patients and the use of fluorochromes for kinetic assays.