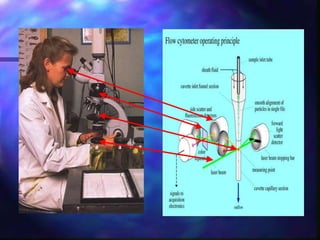
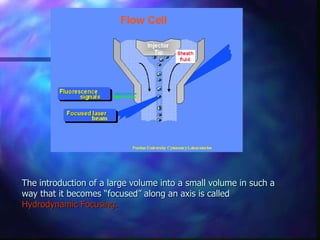
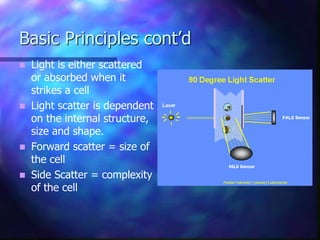
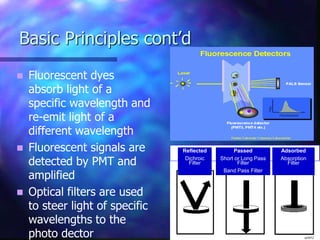
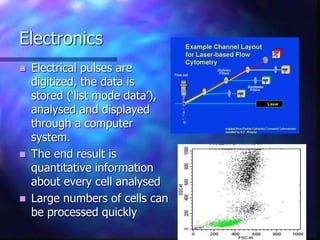
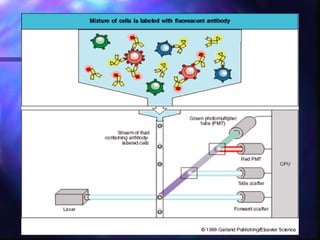
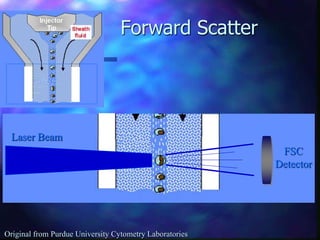
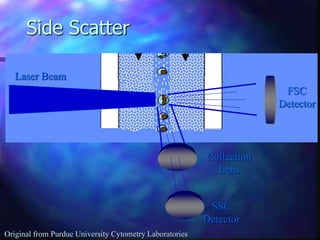
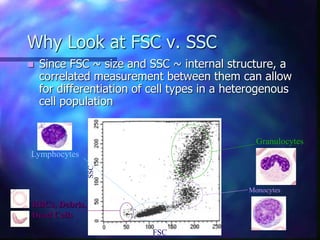
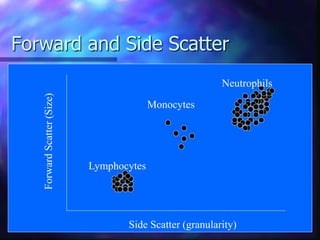
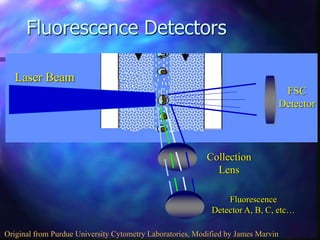
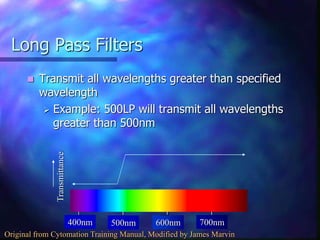
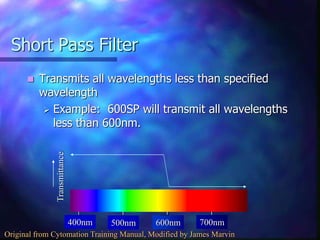
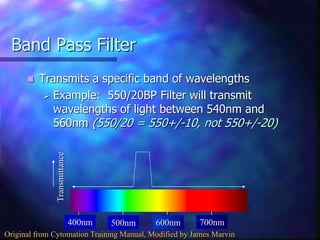
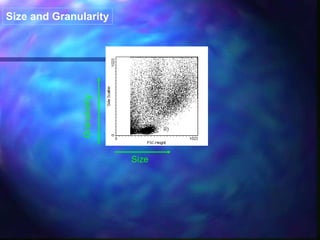
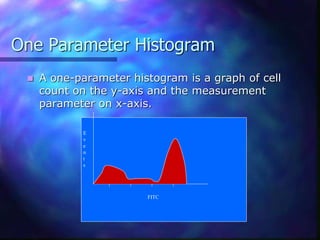
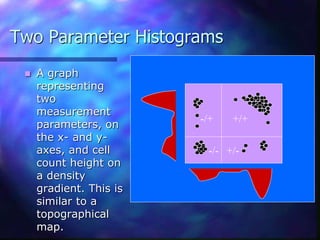
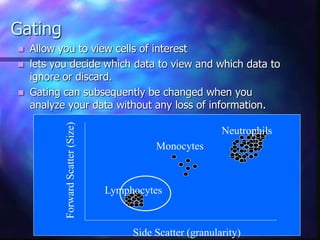
The document provides an overview of the basic principles and components of flow cytometry. It discusses how flow cytometry works by measuring the properties of cells in fluid flow, using a combination of fluidics to introduce cells, optics to generate and collect light signals, and electronics to convert signals to digital data. Key aspects summarized include how cells are hydrodynamically focused and interrogated by light scatter and fluorescence to derive information on their size, granularity, and marker expression that can be analyzed using software.