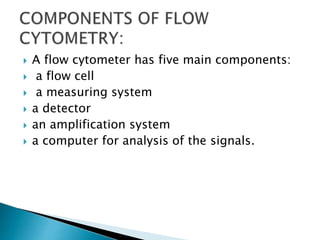
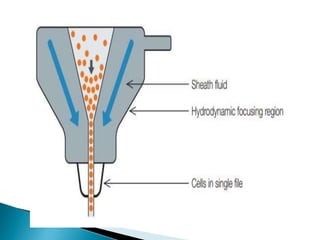
La citometría de flujo es una tecnología que analiza características físicas y químicas de partículas en un fluido al pasar por un láser, permitiendo medir propiedades como granularidad, tamaño e intensidad de fluorescencia de células. Incluye componentes como un sistema de detección, un sistema de amplificación y un software para recolectar y analizar datos, siendo crucial en aplicaciones clínicas y de investigación, especialmente en el diagnóstico de enfermedades de la sangre. También se utiliza en áreas como biología molecular, inmunología y biología marina, contribuyendo al análisis de microorganismos y variantes de proteínas superficiales en células.