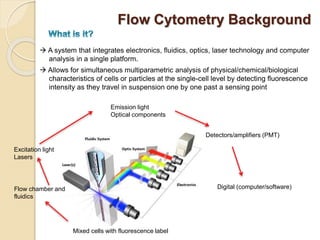
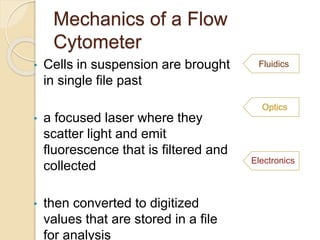
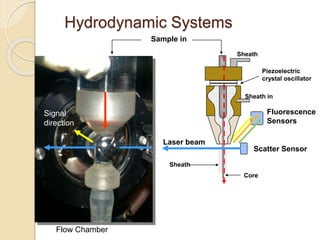
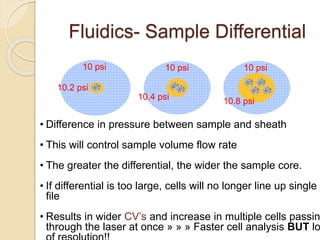
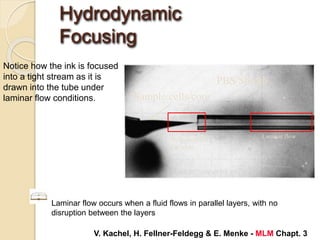
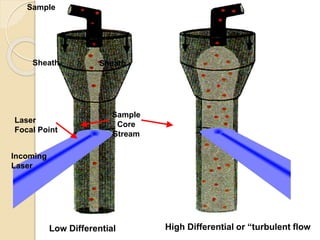
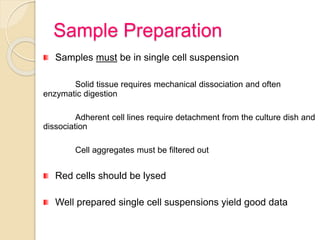
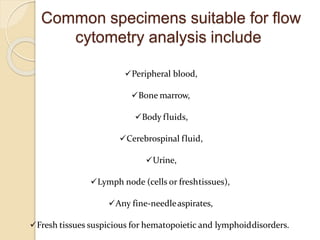
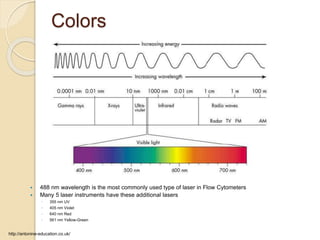
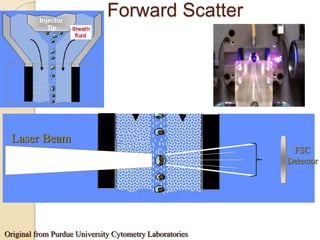
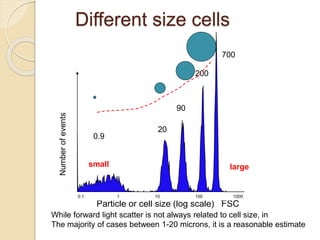
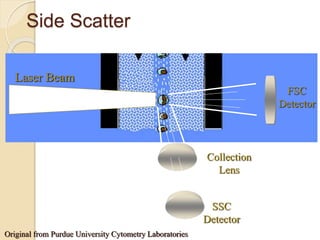
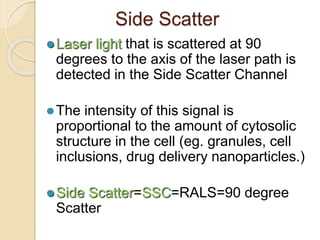
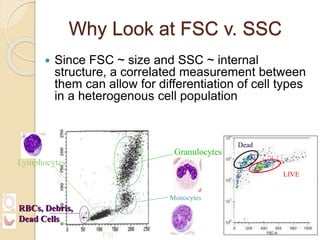
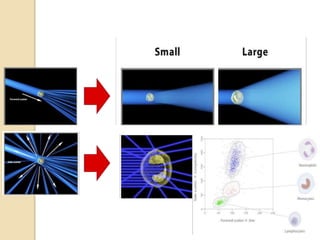
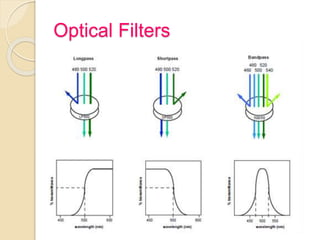
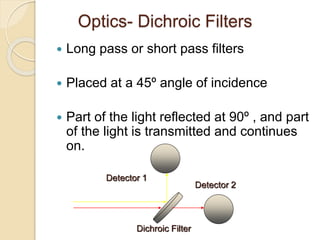
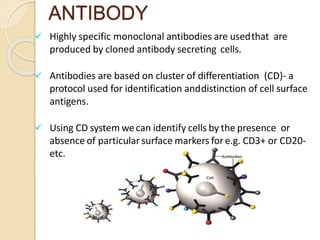
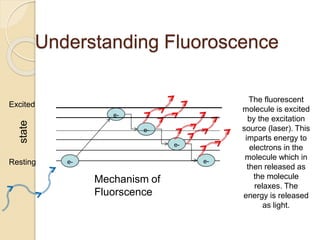
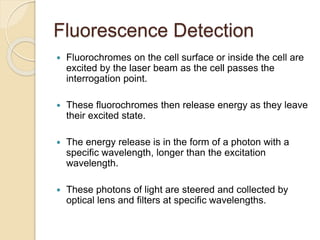
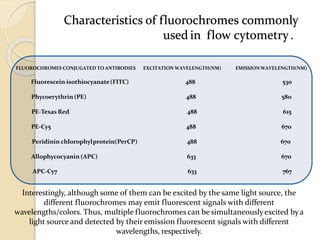
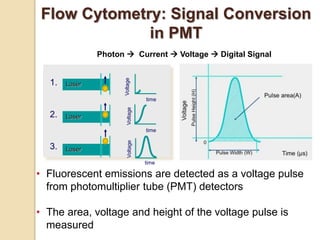
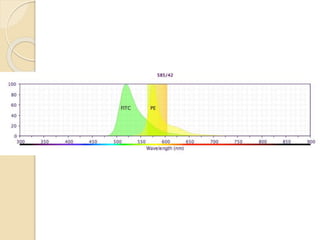
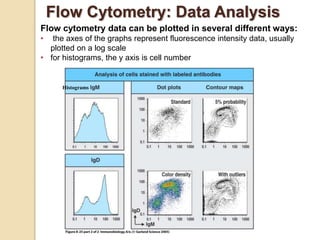
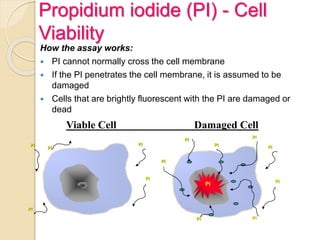
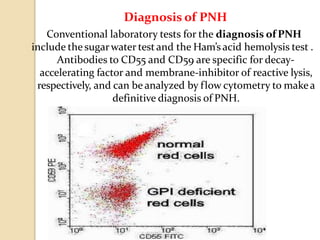
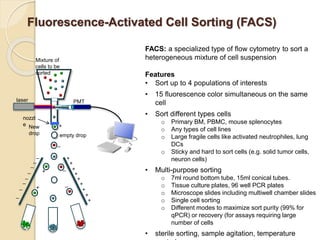
Flow cytometry is a technology that combines fluidics, optics, electronics, and computer analysis, allowing for the simultaneous analysis of multiple cellular characteristics at the single-cell level. Its development began in the late 19th century and has evolved significantly, enabling applications in clinical laboratories such as immunophenotyping, leukemia detection, and organ transplantation. Key advancements include the introduction of lasers for detecting fluorescence and the use of monoclonal antibodies for specific cell identification.