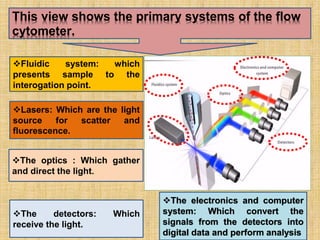
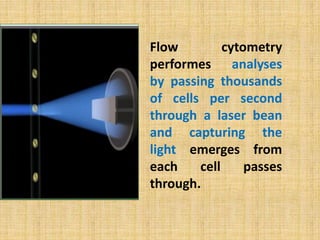
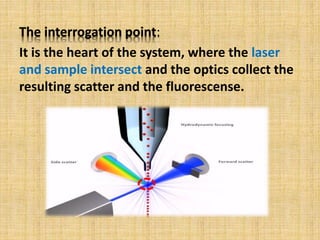
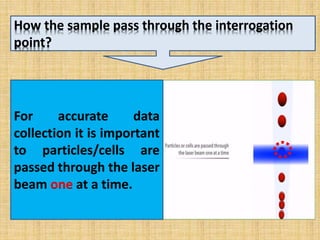
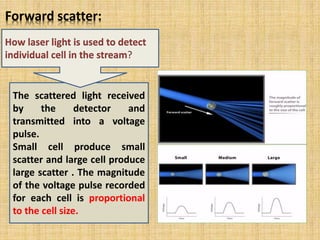
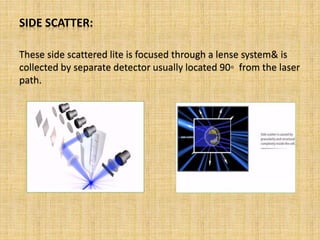
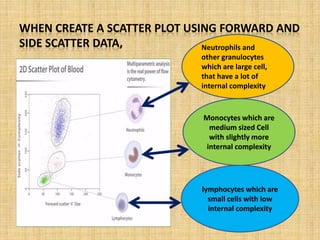
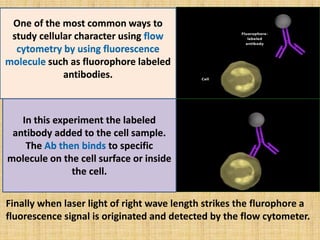
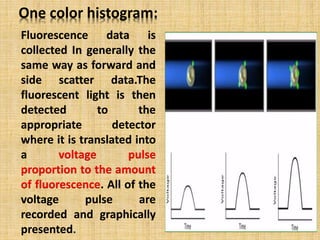
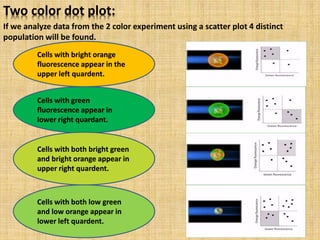
The document discusses flow cytometry, a laser-based technology for measuring various properties of single cells in a fluid stream. It outlines the components of a flow cytometer, the principles of operation, and different applications such as immunophenotyping, DNA content analysis, cell cycle analysis, and apoptosis detection. Additionally, it highlights techniques involved in fluorescence detection and data analysis, including histograms and scatter plots.