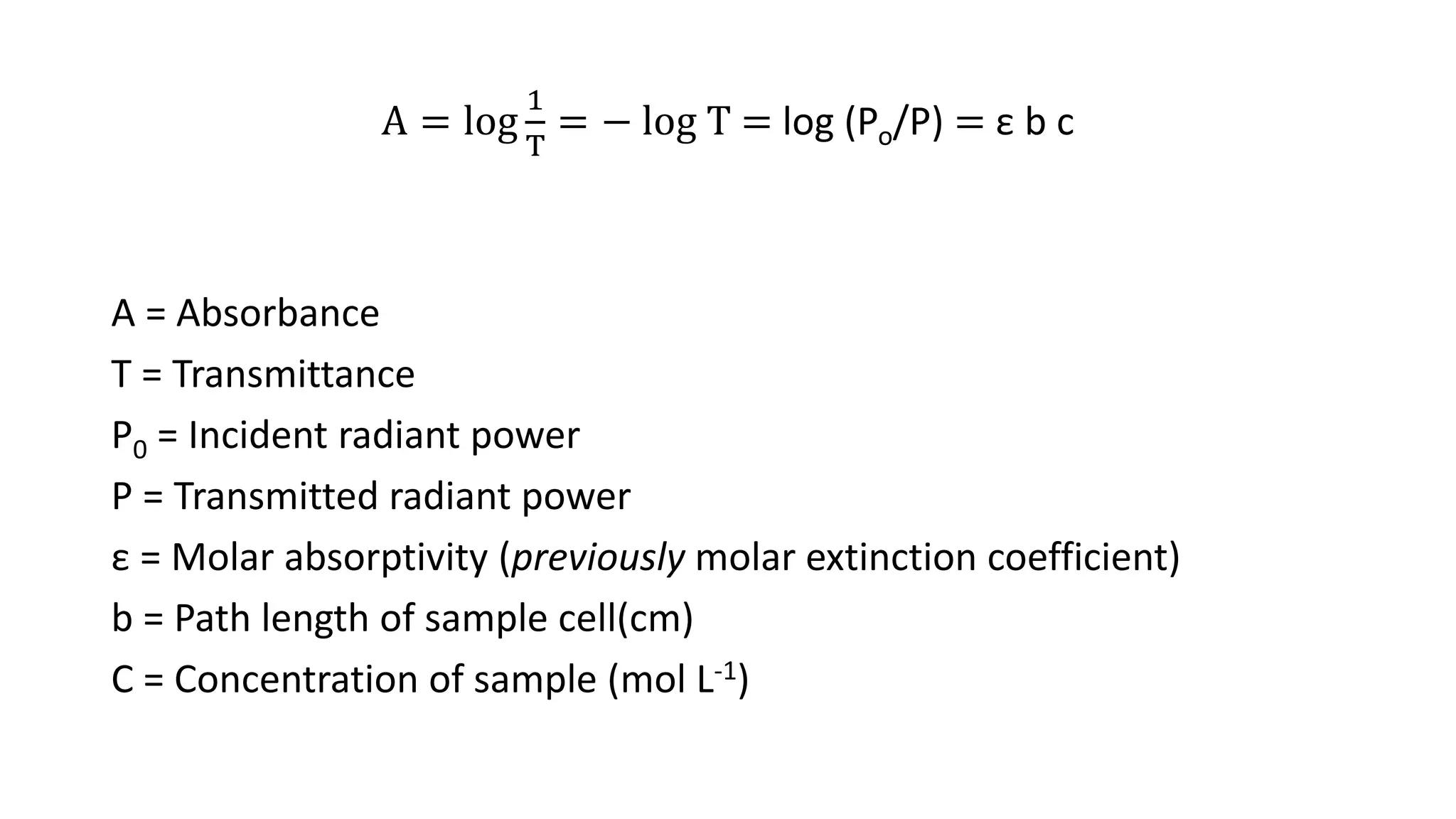
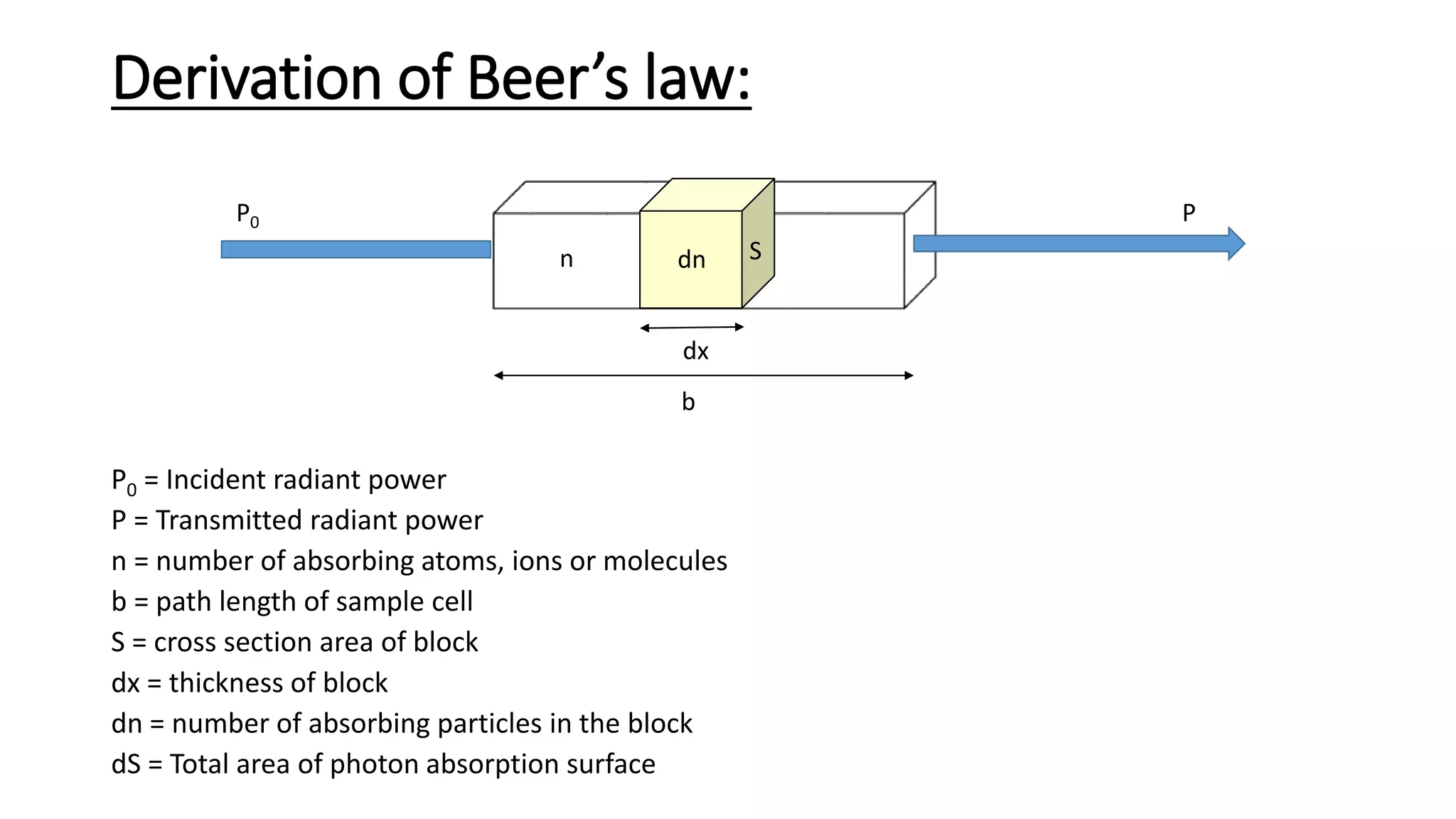
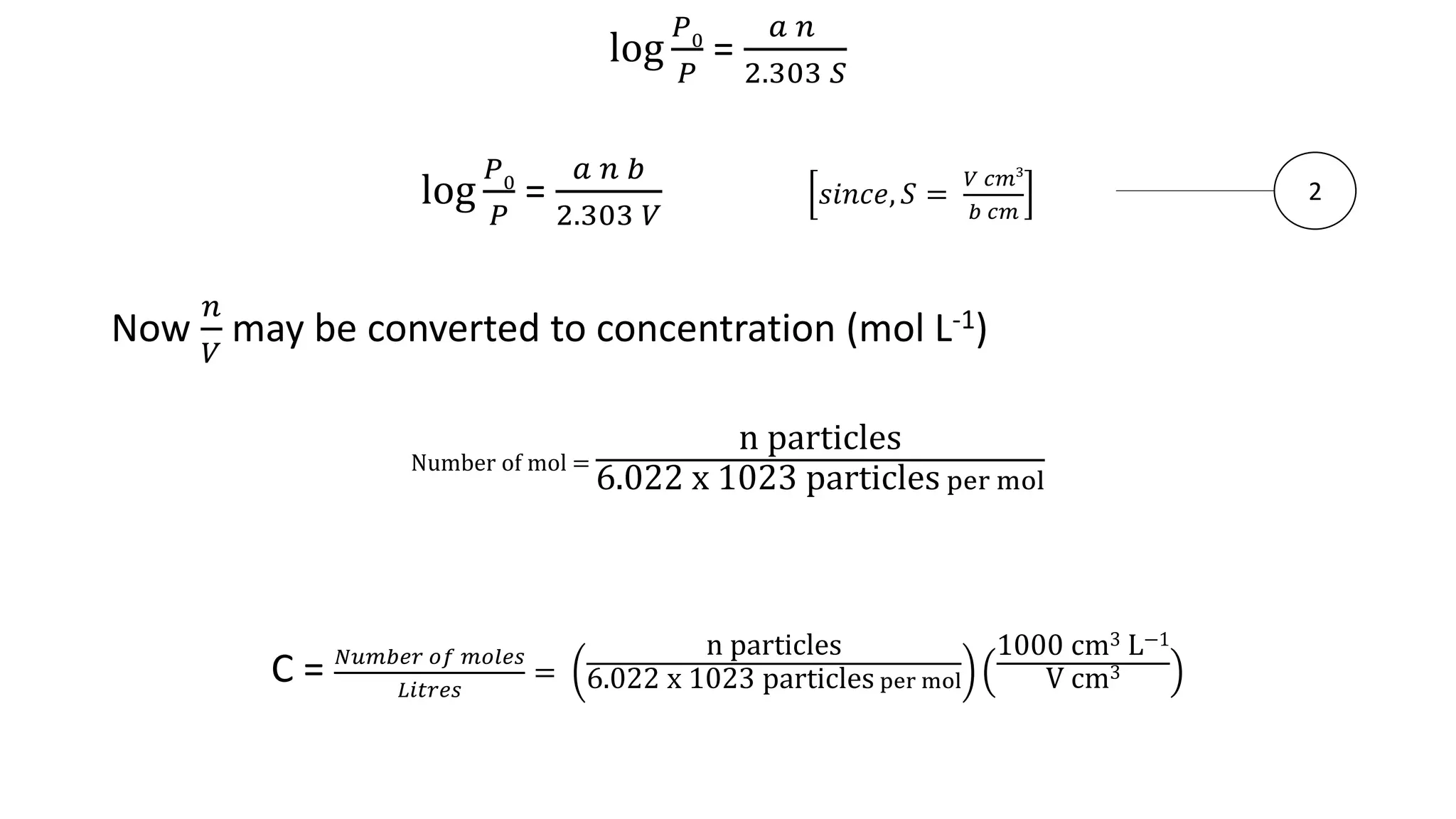
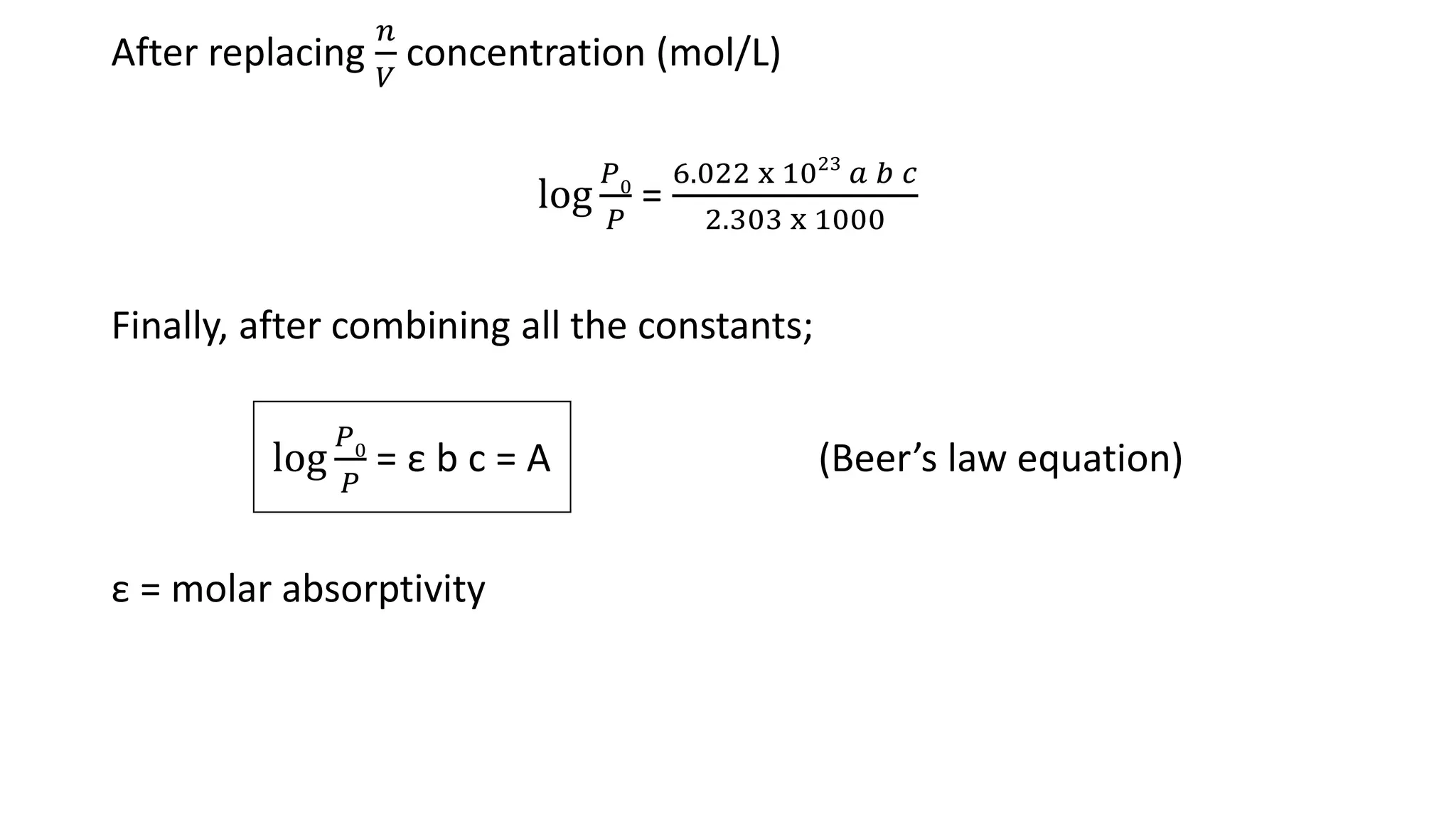
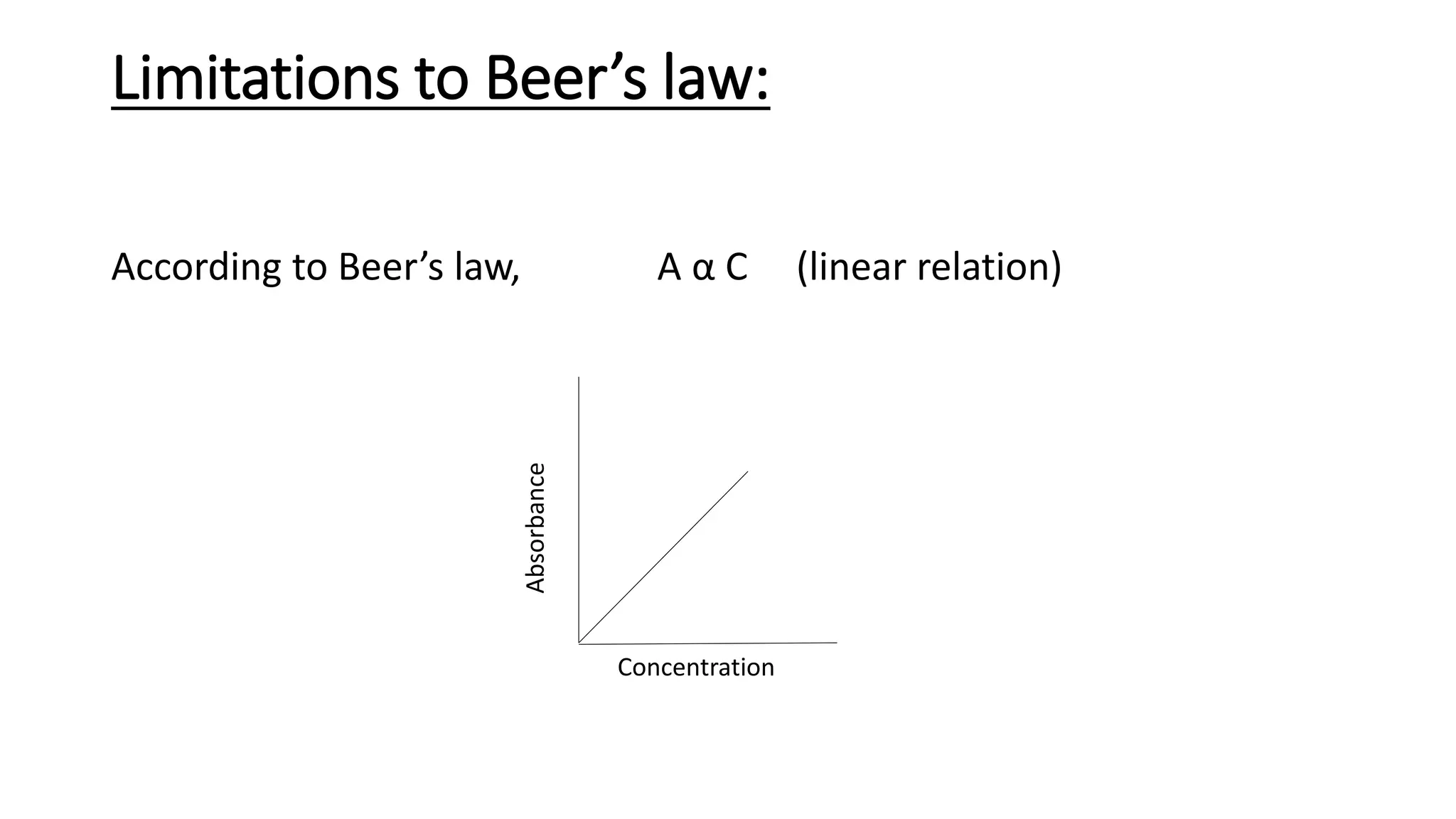
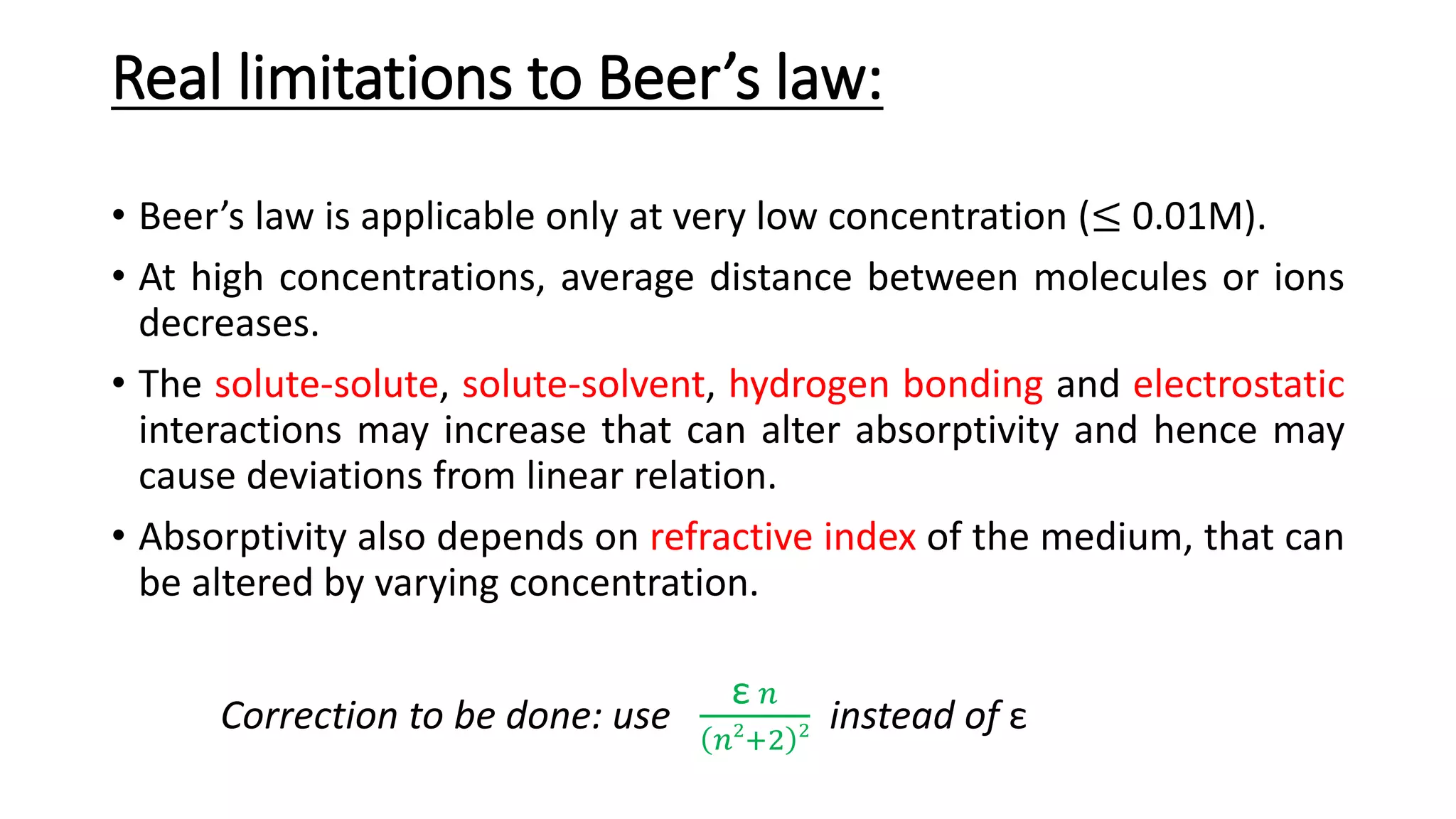
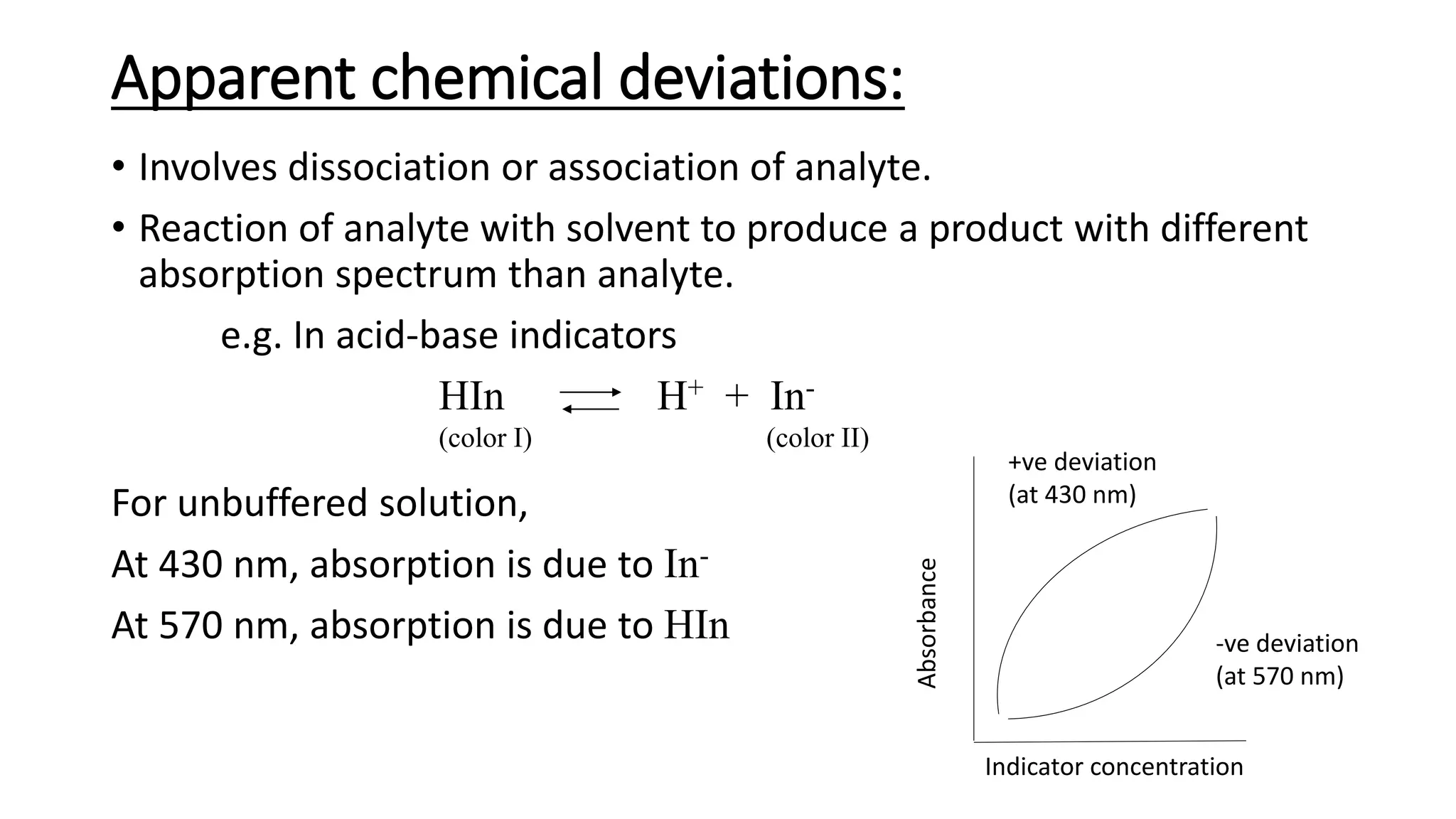
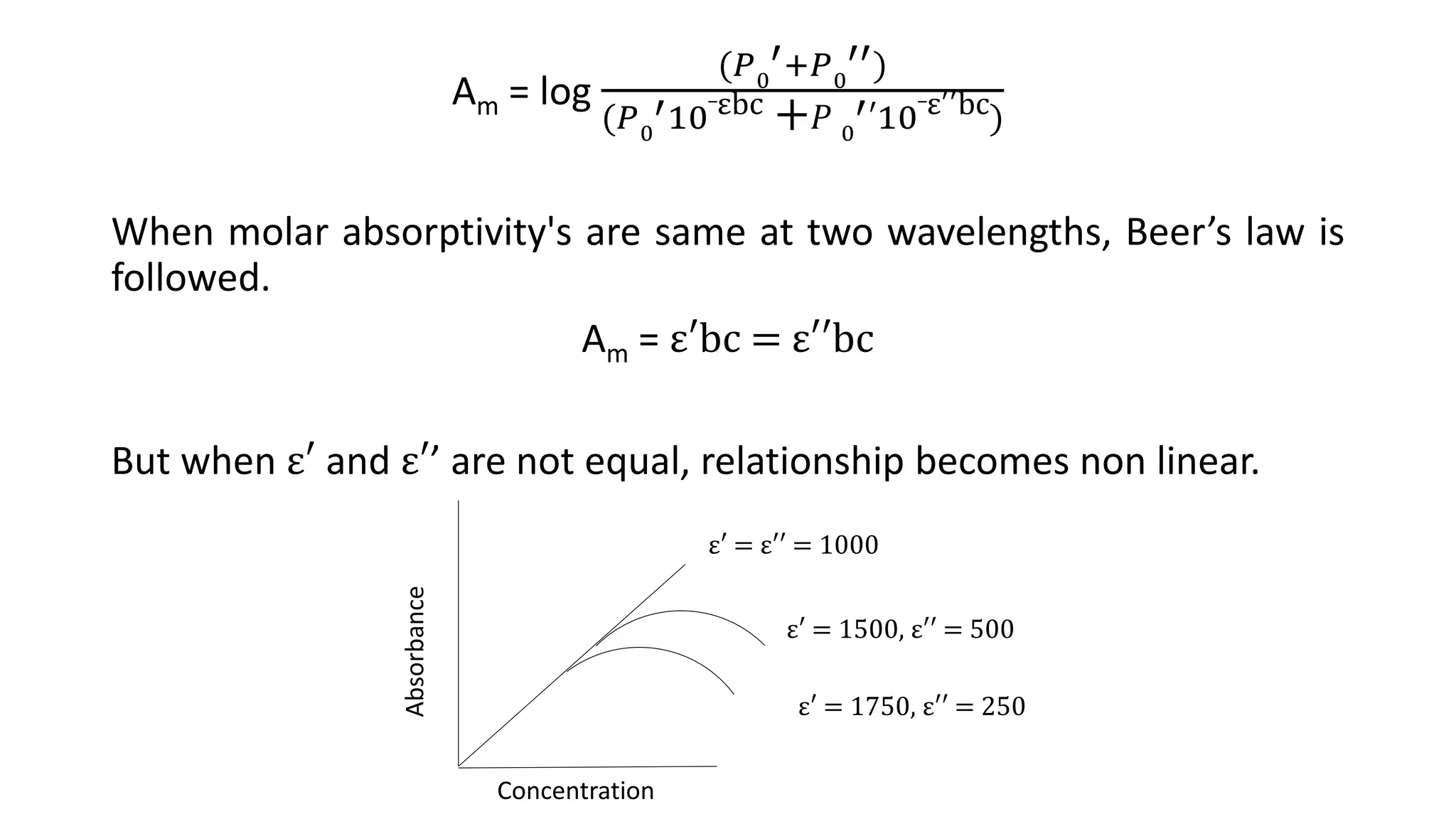
This document discusses Beer's law, which states that absorbance of a solution is directly proportional to the concentration of the absorbing material in the solution. It defines Beer's law, derives the mathematical equation, and lists some limitations and sources of deviation from the law, including high concentrations, dissociation/association reactions, use of polychromatic radiation, stray light, and mismatched sample cells. The derivation shows how the Beer's law equation is obtained based on probability of photon absorption in thin sections of the sample.