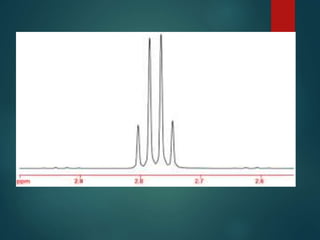
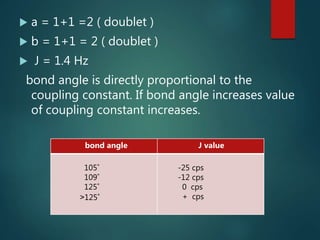
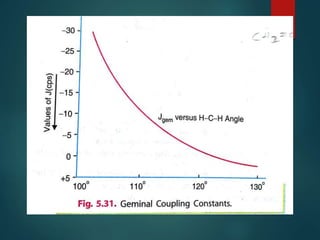
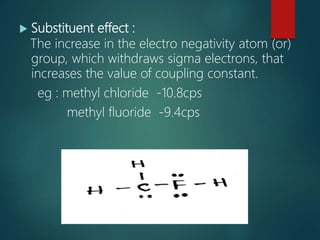
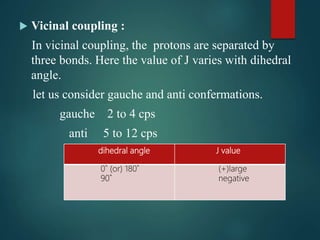
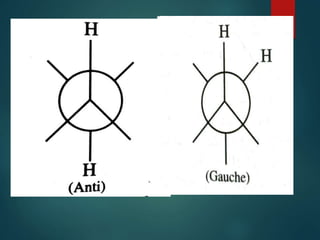
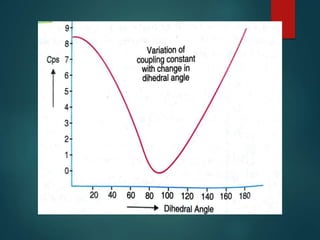
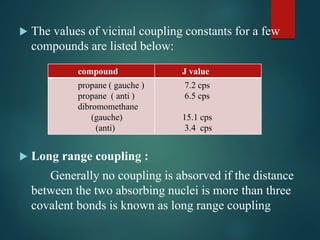
The coupling constant is the distance between peaks in a multiplet in NMR spectroscopy. It is measured in Hertz and does not depend on external magnetic field strength. The value of the coupling constant provides information to distinguish multiplets and can indicate structural features like cis/trans isomers. Coupling occurs between protons close in space, known as geminal, vicinal, and sometimes long-range coupling over several bonds. The coupling constant is affected by factors like bond angle, dihedral angle, and electronegativity of substituents.