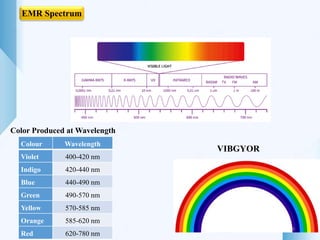
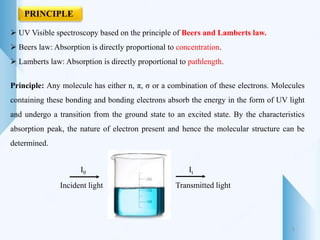
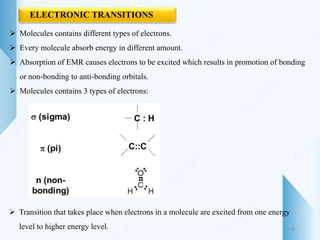
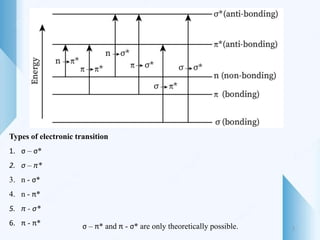
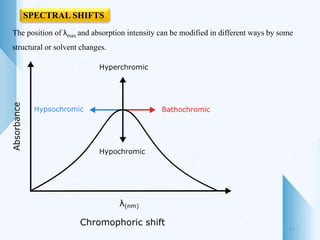
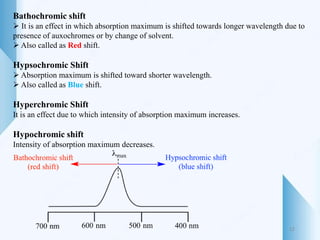
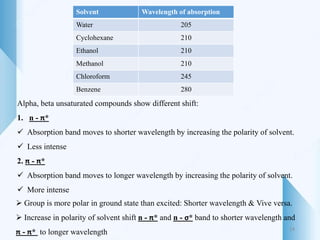
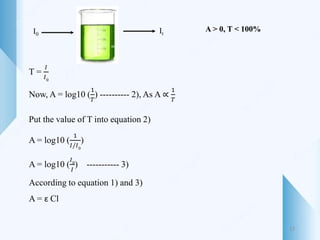
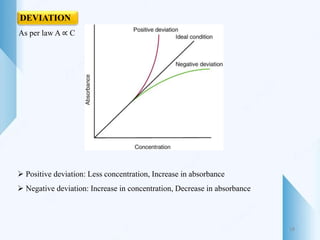
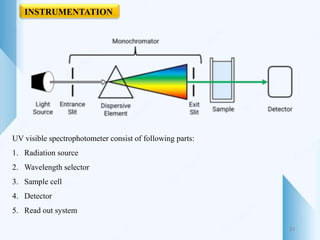
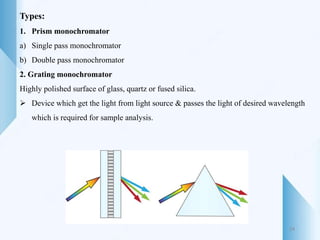
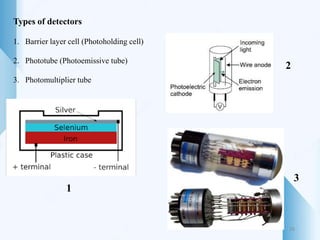
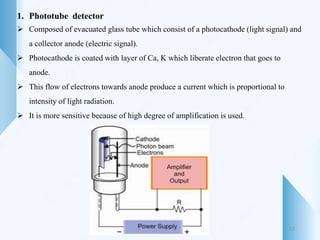
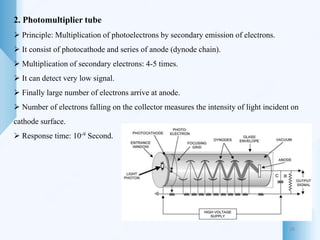
UV-visible spectroscopy involves using light in the UV-visible spectral region to analyze chemical substances. It works on the principle of Beer-Lambert's law, where absorbance is directly proportional to concentration and path length. Different functional groups and conjugated systems can absorb light at characteristic wavelengths. The technique is used for quantitative and qualitative analysis of samples through measurement of absorption spectra. It provides information about electronic transitions and molecular structure of compounds.