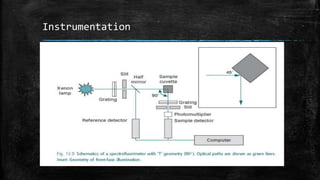
Fluorescence spectroscopy involves three main processes: excitation, where a molecule absorbs a photon and reaches an excited state; internal conversion and vibrational relaxation in the excited state; and fluorescence emission, where the molecule returns to the ground state and emits a photon. It has many applications including structural elucidation of molecules, monitoring molecular interactions and conformational changes, and tracking ions and biomolecules in cells. Specifically, intrinsic protein fluorescence relies on tryptophan residues, while extrinsic labels are often used for non-fluorescent compounds. Fluorescence resonance energy transfer (FRET) also allows measuring distances between fluorophores to study biomolecular interactions and conformational dynamics.