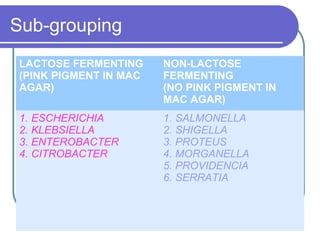
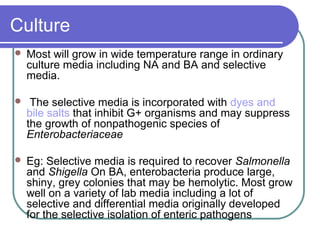
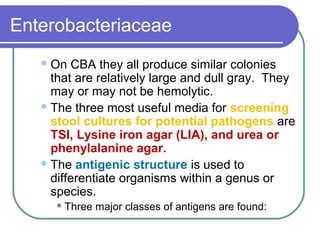
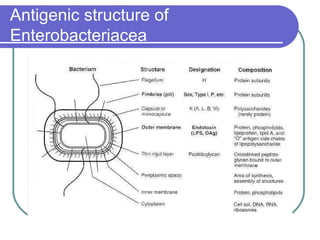
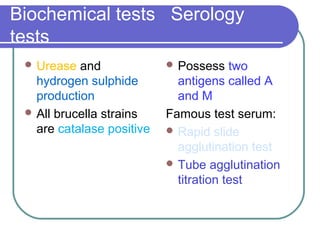
The document summarizes the Enterobacteriaceae family of bacteria. It describes their morphology as gram negative rods that are facultative anaerobes. It discusses their sub-grouping based on lactose fermentation and pigment production. It also outlines their culture characteristics, biochemical reactions, antigenic structures, and important genera like Escherichia, Klebsiella, Salmonella and Shigella. Selective media and differential tests help identify different members of the family.