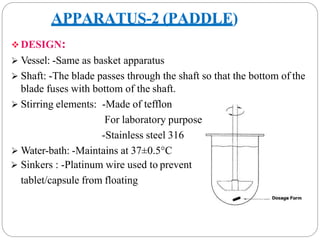
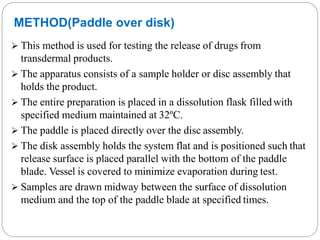
The document discusses dissolution as a tool in pharmaceutics. It defines dissolution as the process where a solid substance solubilizes in a solvent, transferring from the solid surface to the liquid phase. This is the rate-determining step for poorly soluble drugs. The document discusses three main mechanisms of dissolution - the diffusion layer model, Danckwert's model, and the interfacial barrier model. It also covers factors influencing dissolution such as drug properties, apparatus factors, and dissolution media properties. Finally, it provides details on three common dissolution apparatus - the basket, paddle, and reciprocating cylinder methods.