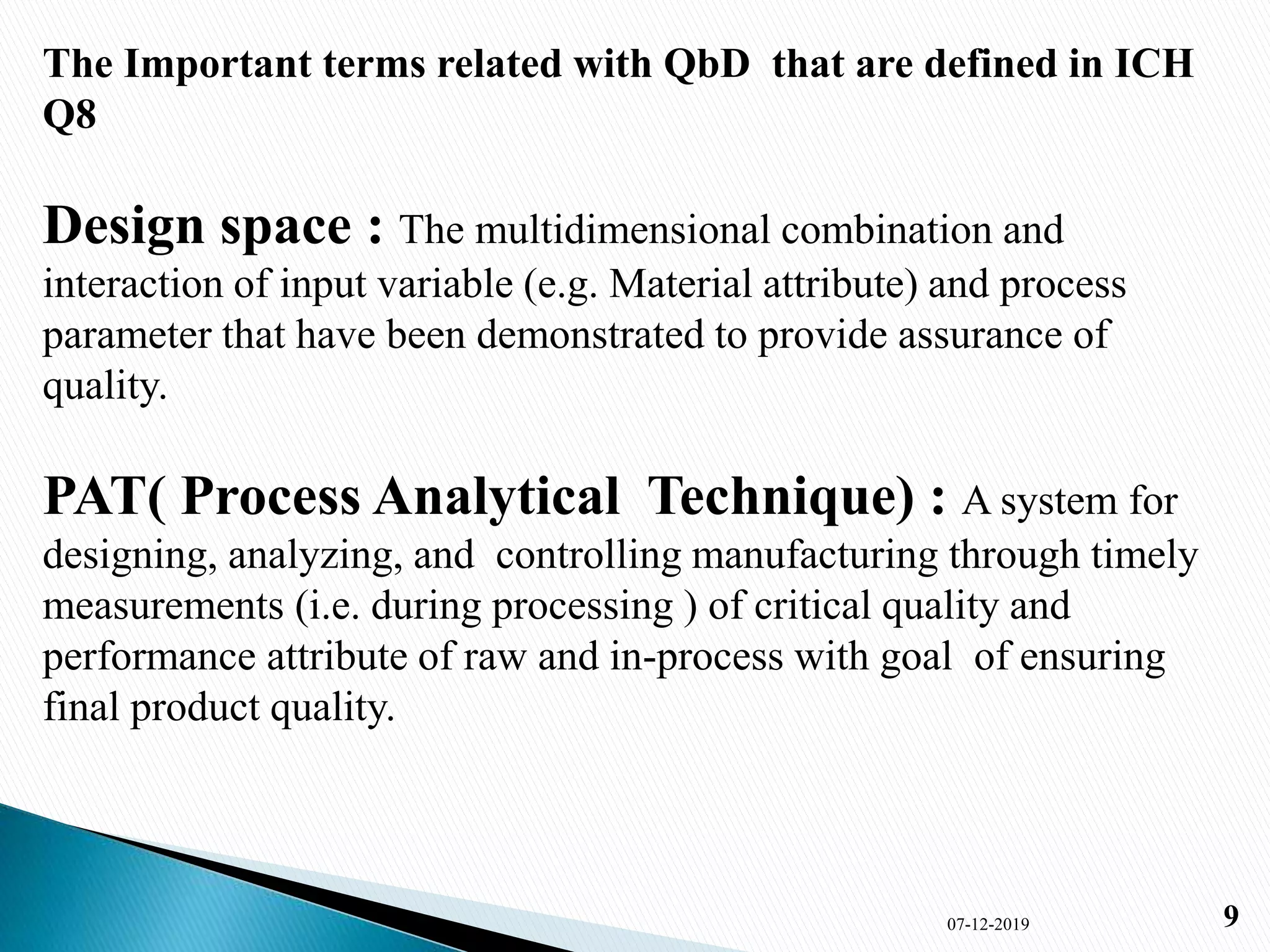
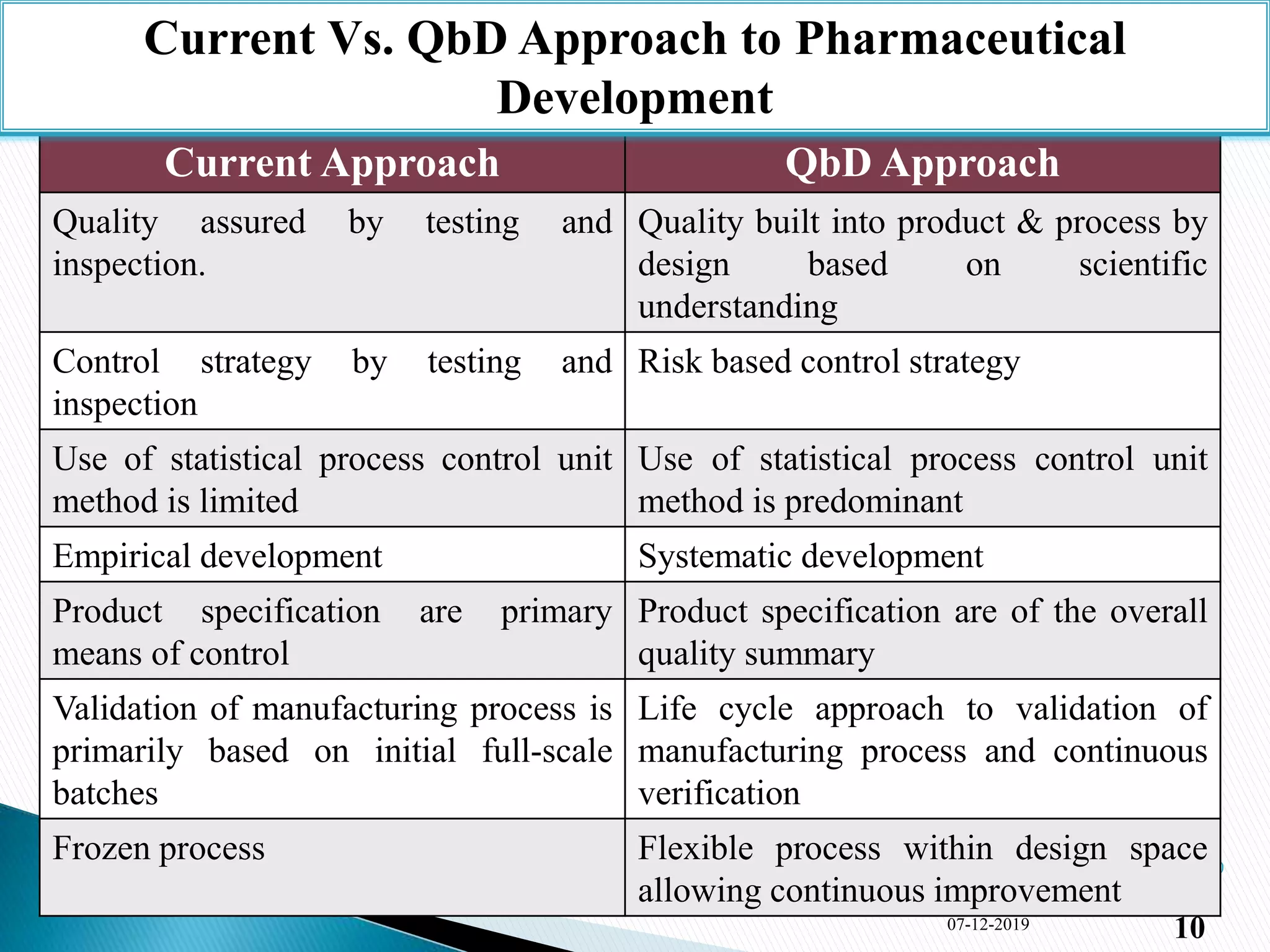
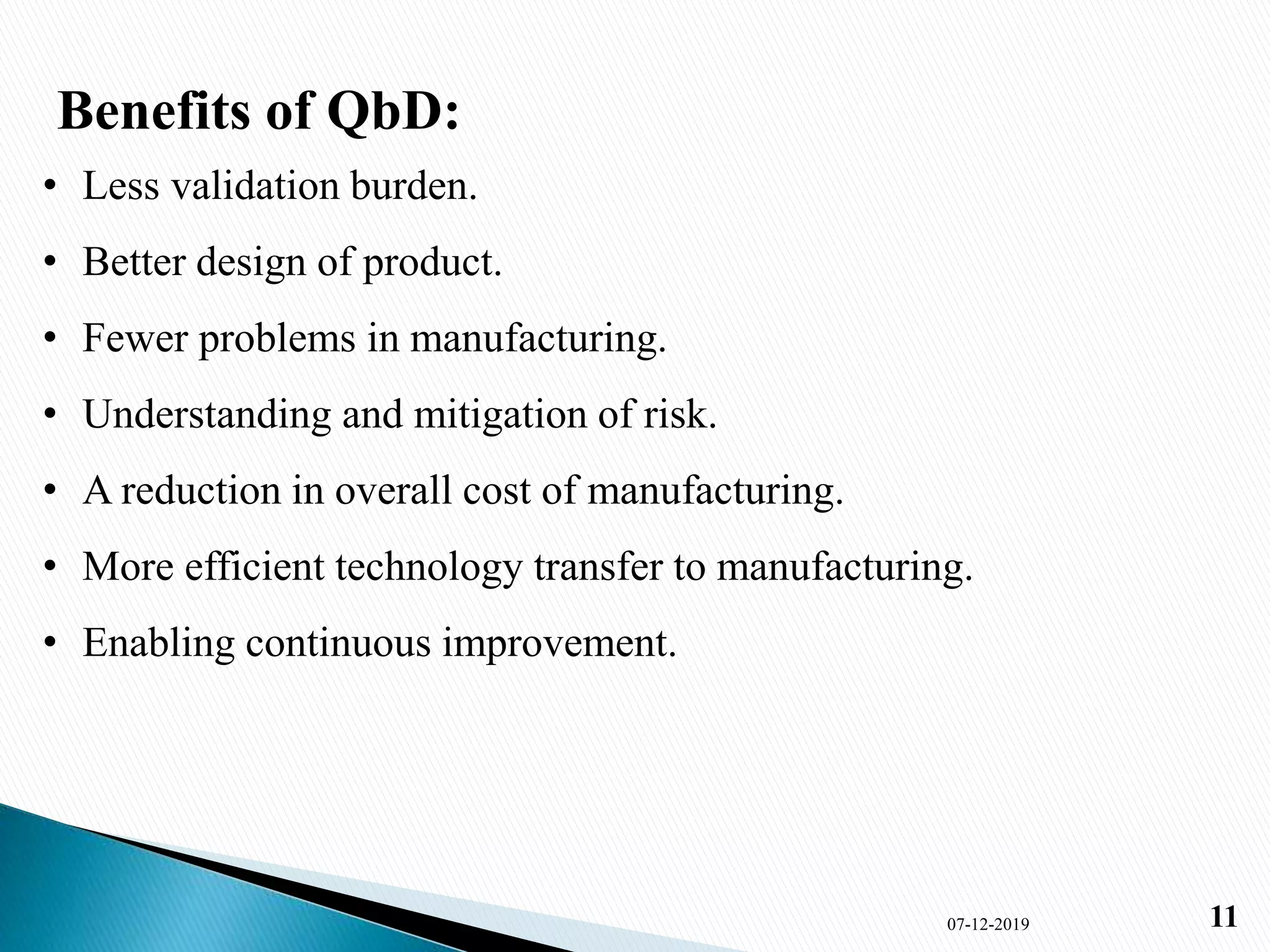
1) The document presents an overview of Quality by Design (QbD) in pharmaceutical development. It defines QbD, compares the current and QbD approaches, and outlines the benefits, objectives, and elements of QbD.
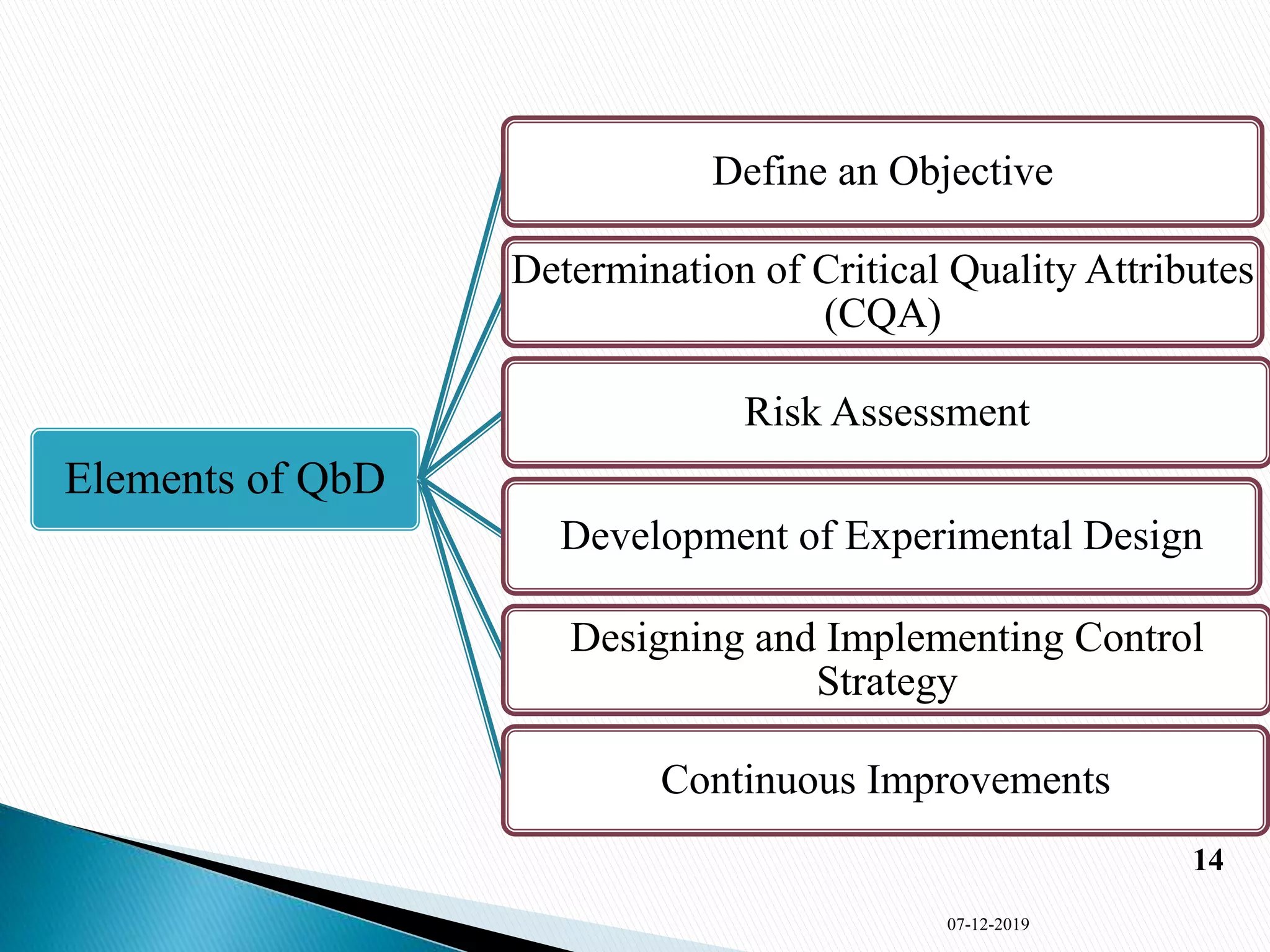
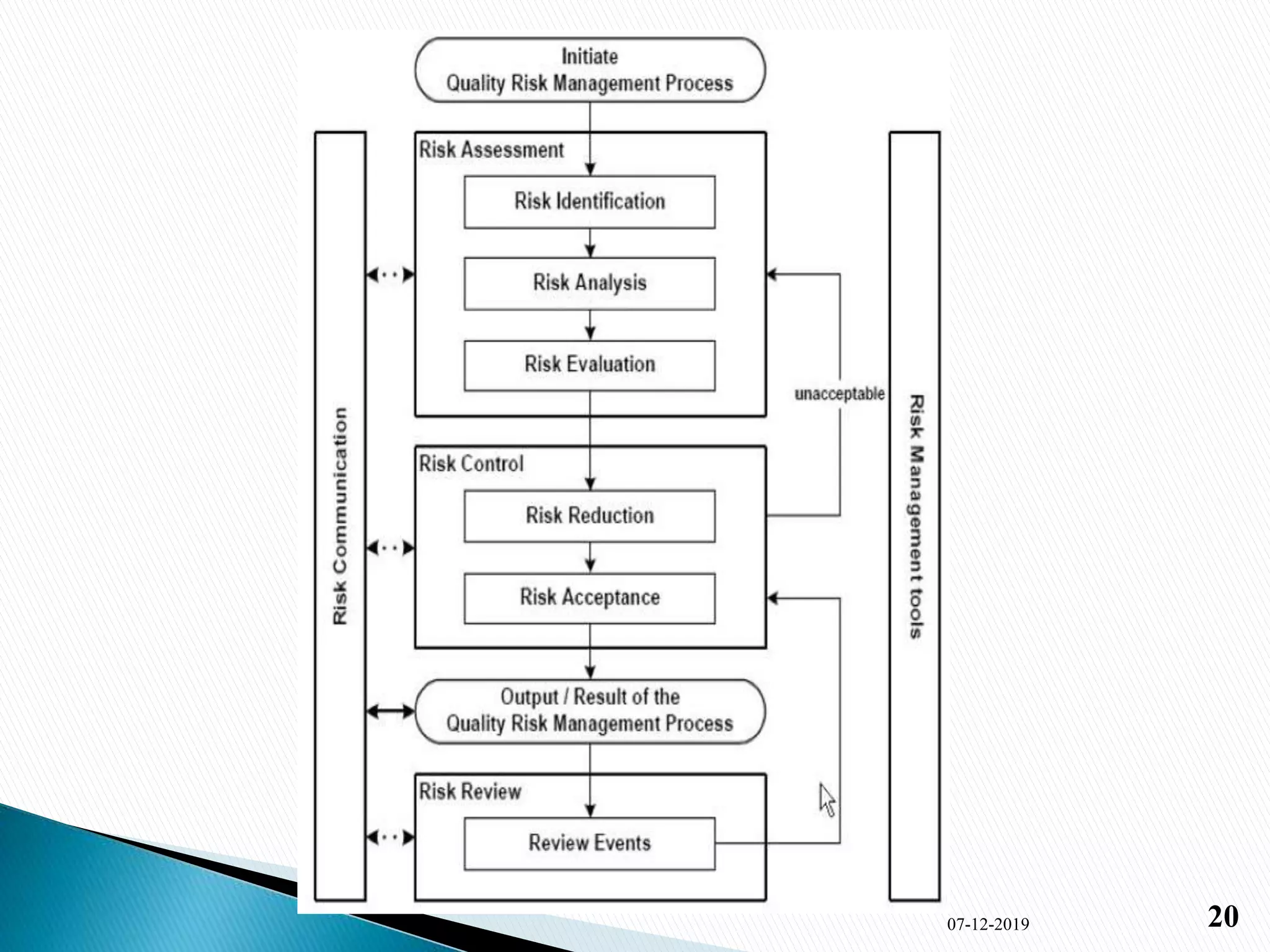
2) The key elements of QbD discussed are defining objectives, determining critical quality attributes, risk assessment, experimental design, control strategy, and continuous improvement. Ishikawa and risk assessment methods are also summarized.
3) Implementing QbD provides quality medicines to patients, production improvements for manufacturers, and greater confidence for drug regulators by ensuring predefined product quality objectives.