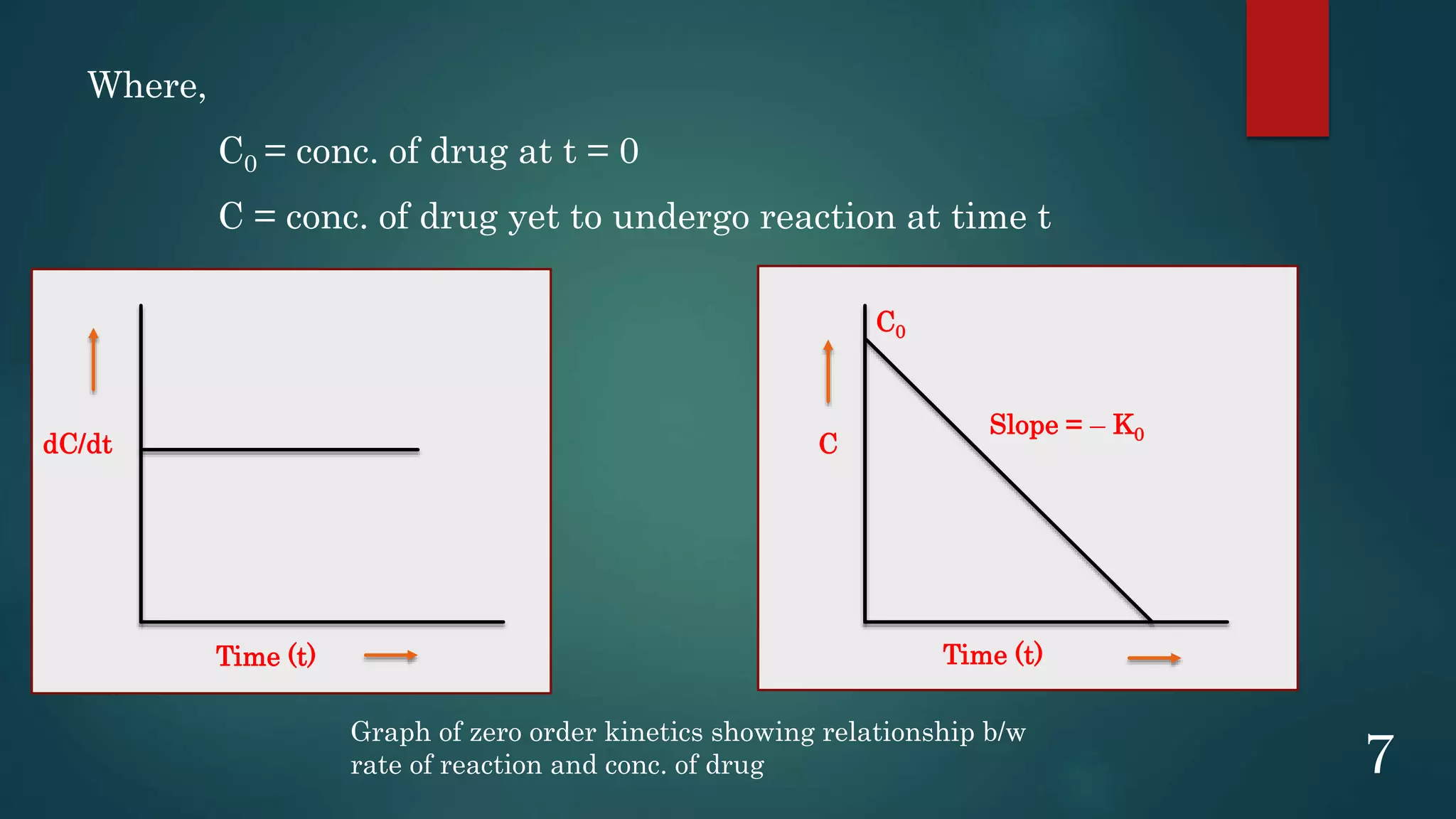
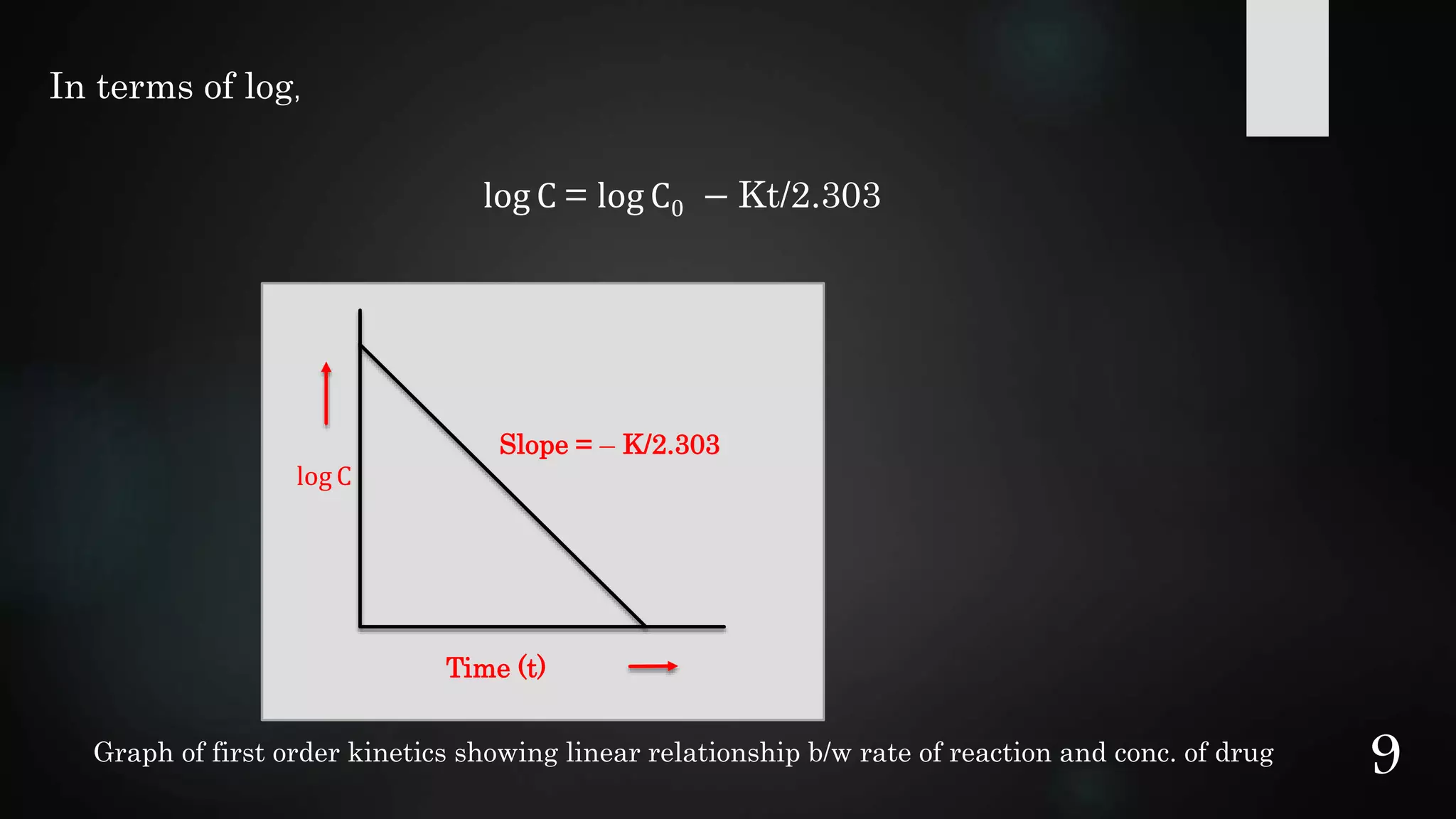
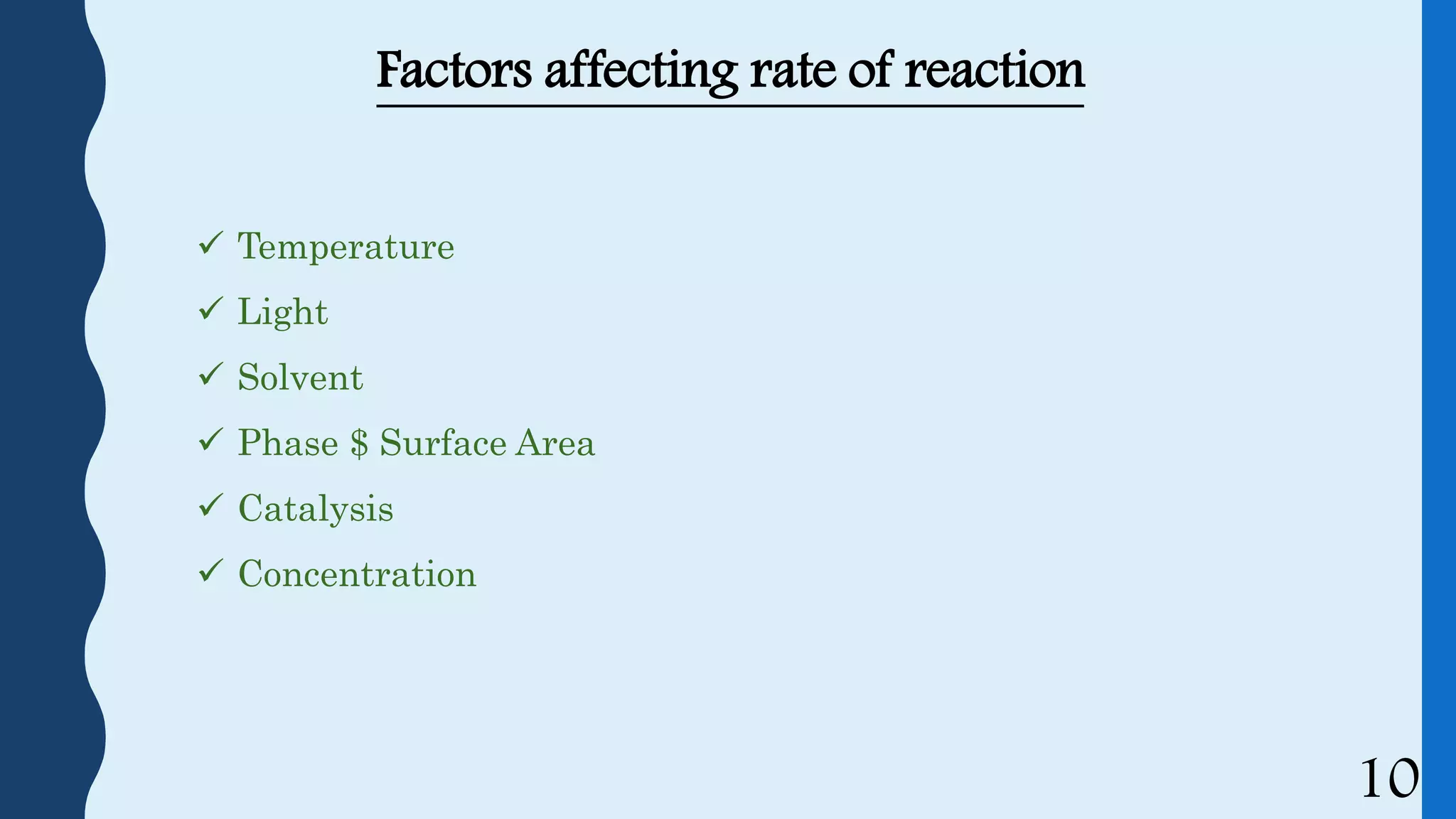
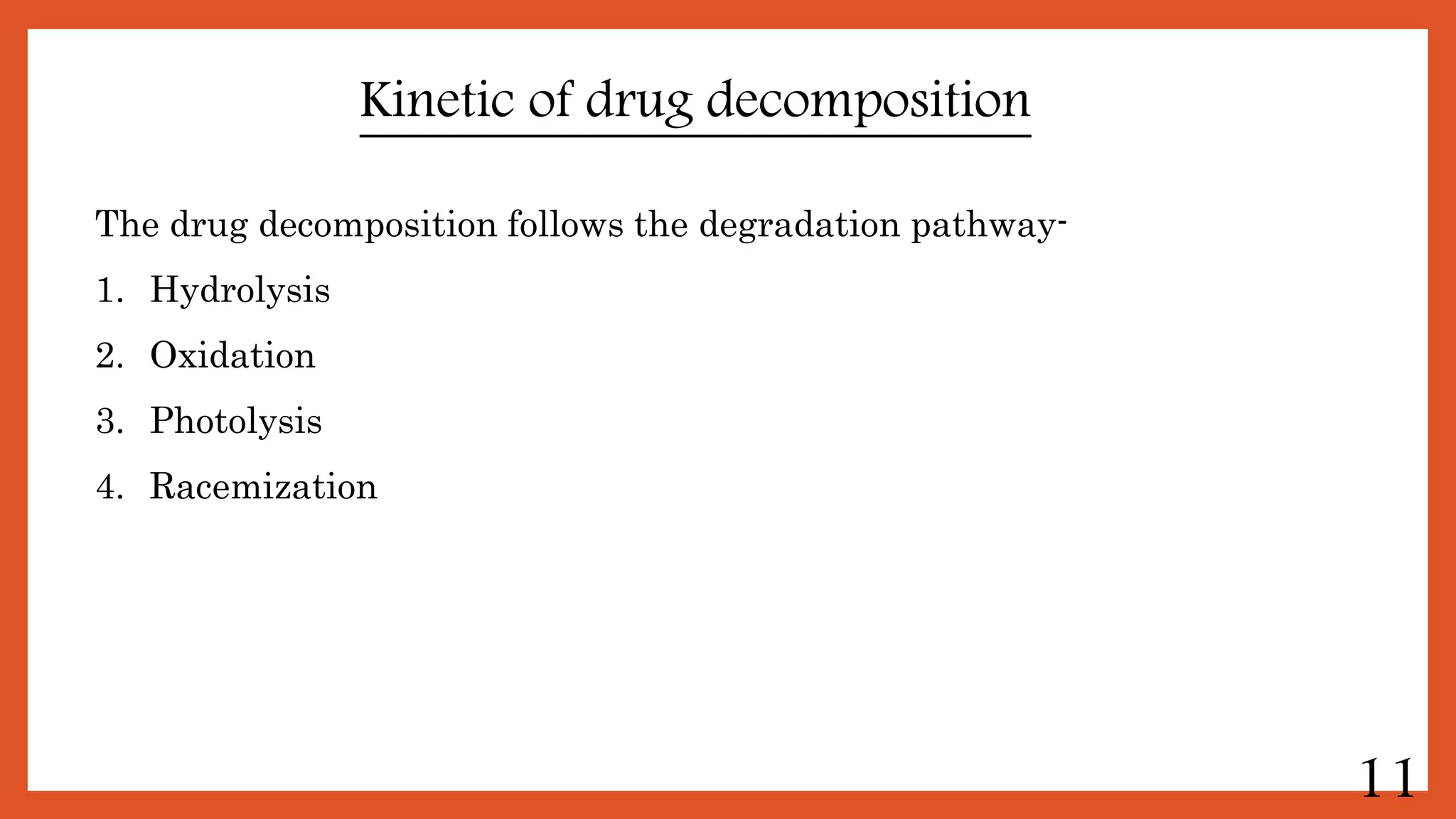
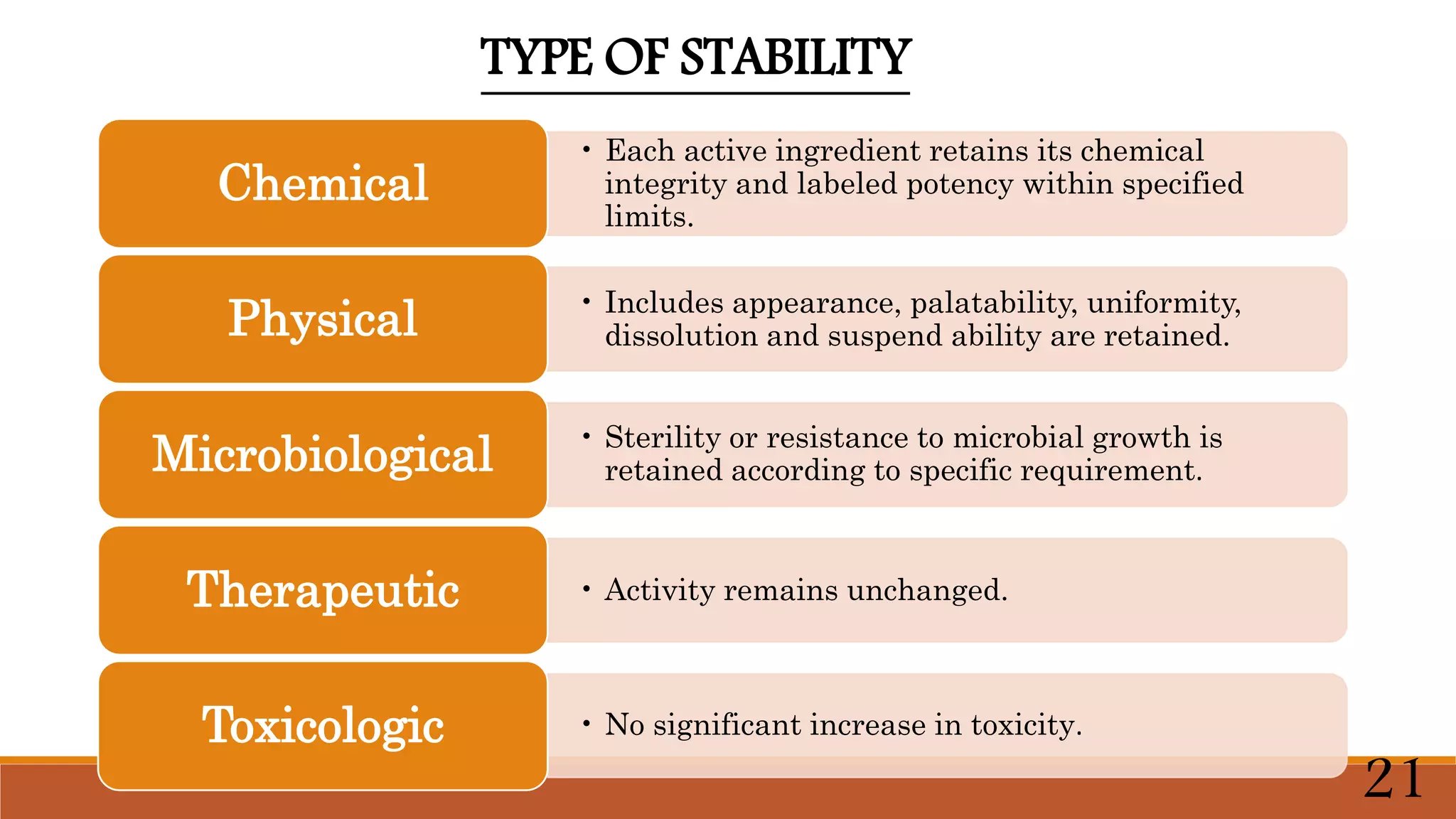
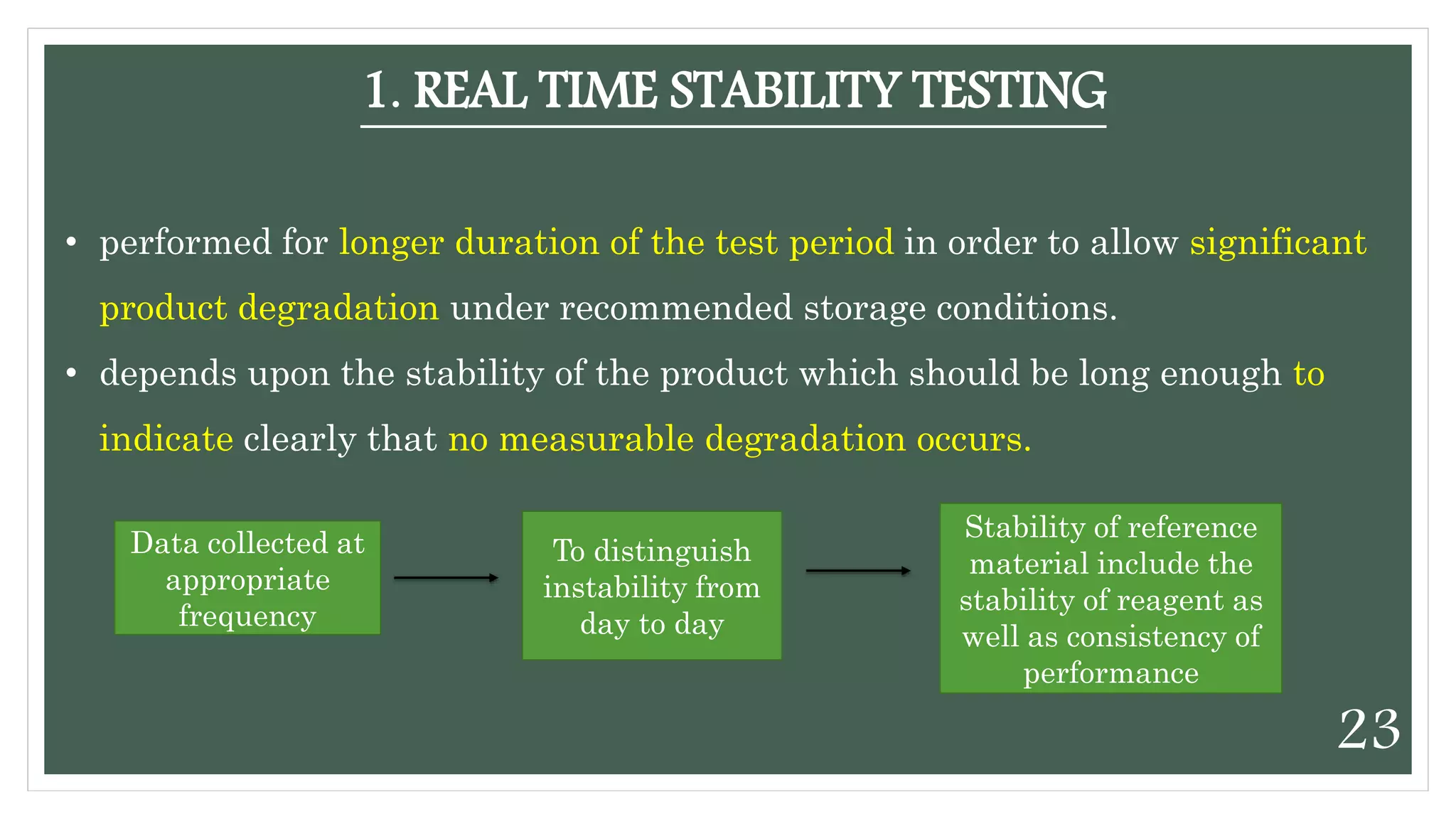
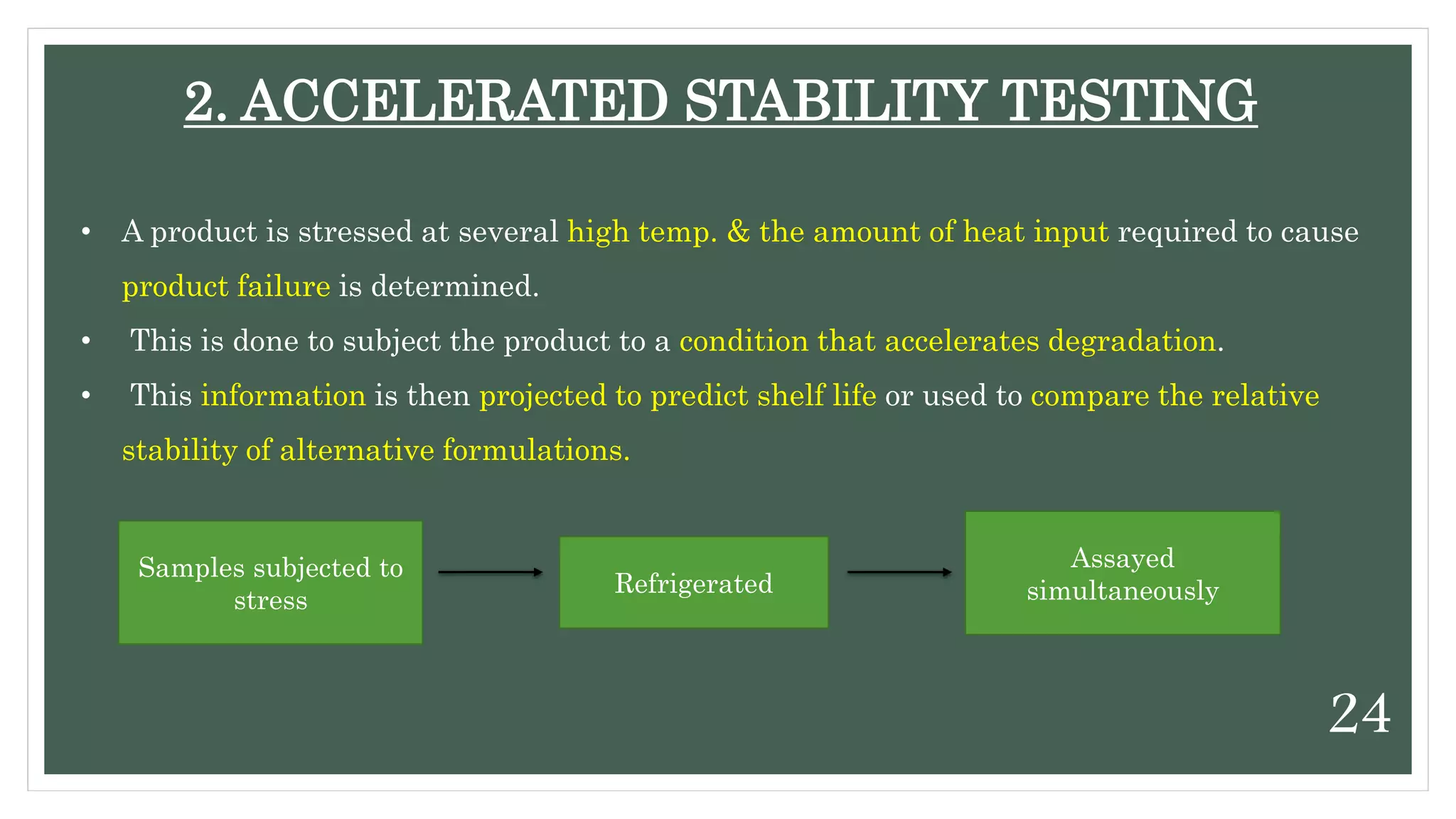
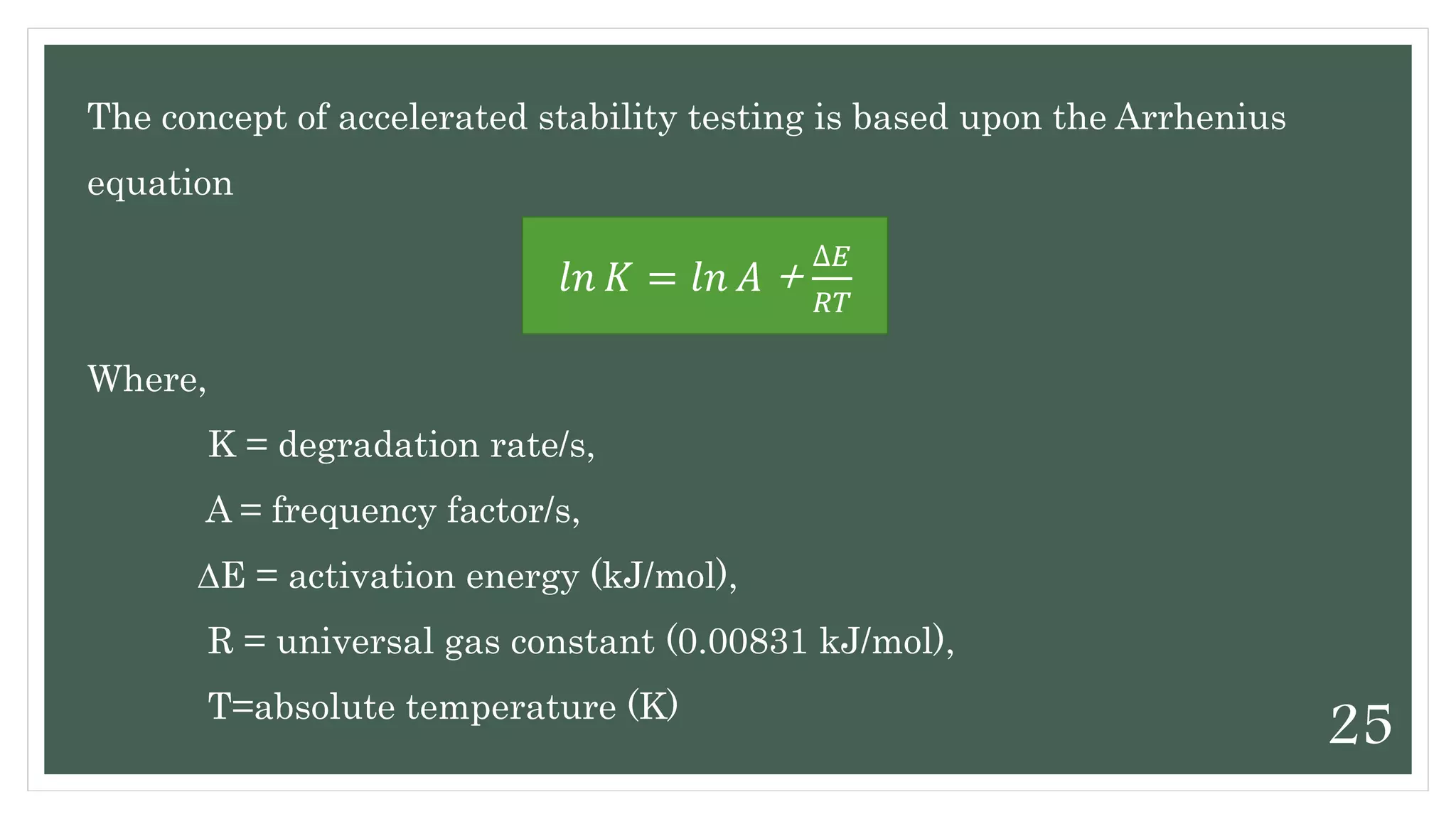
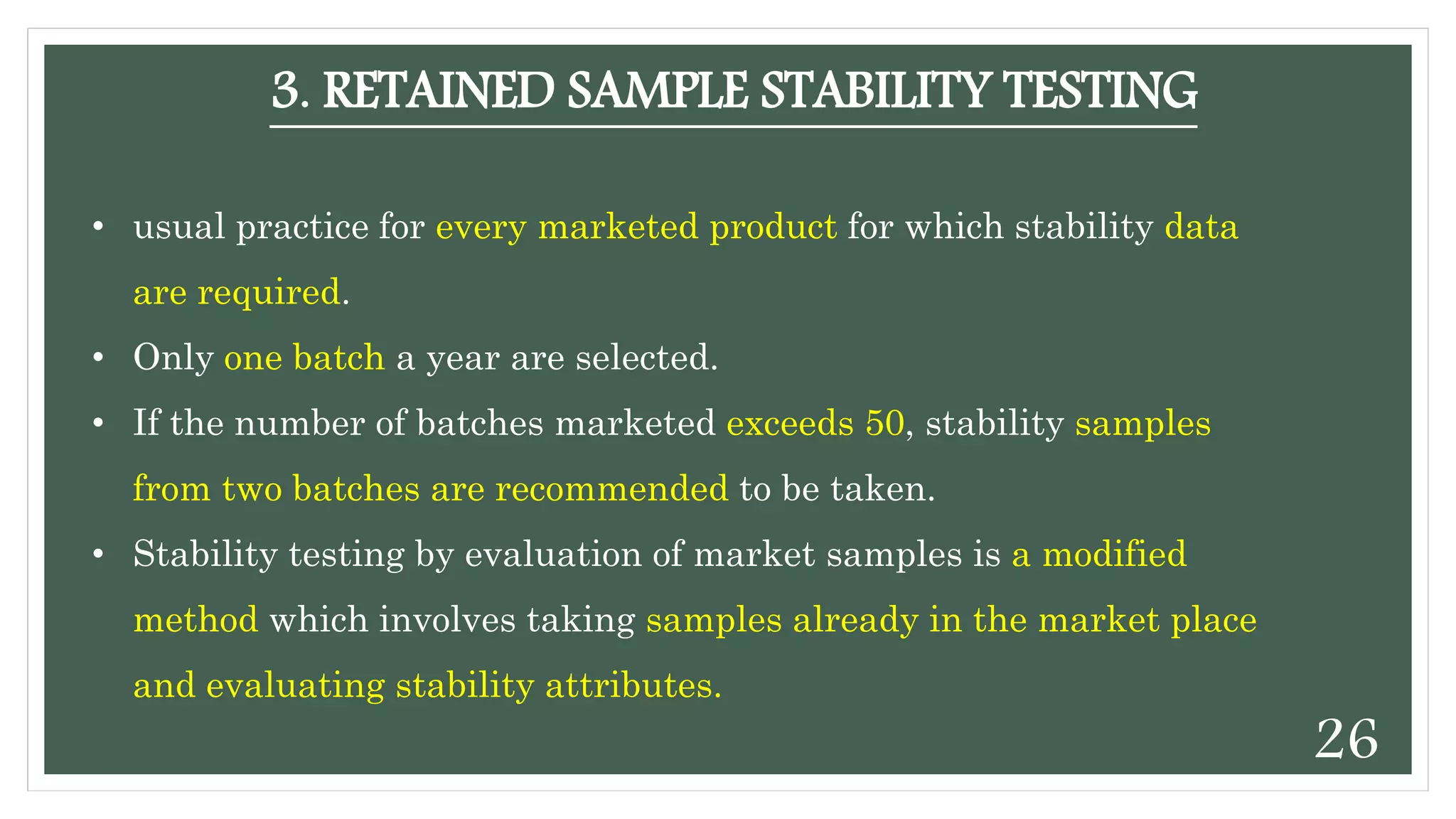
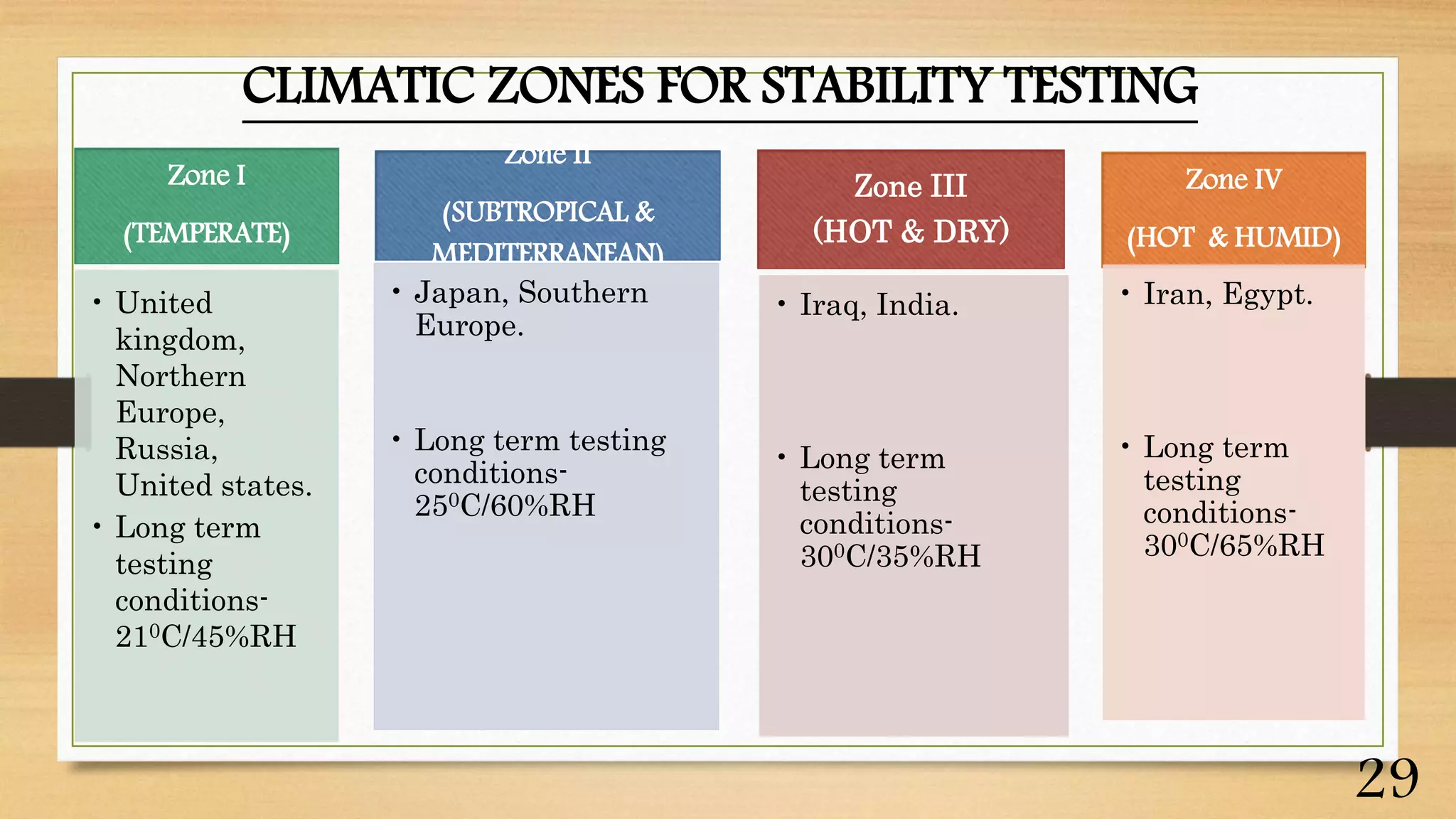
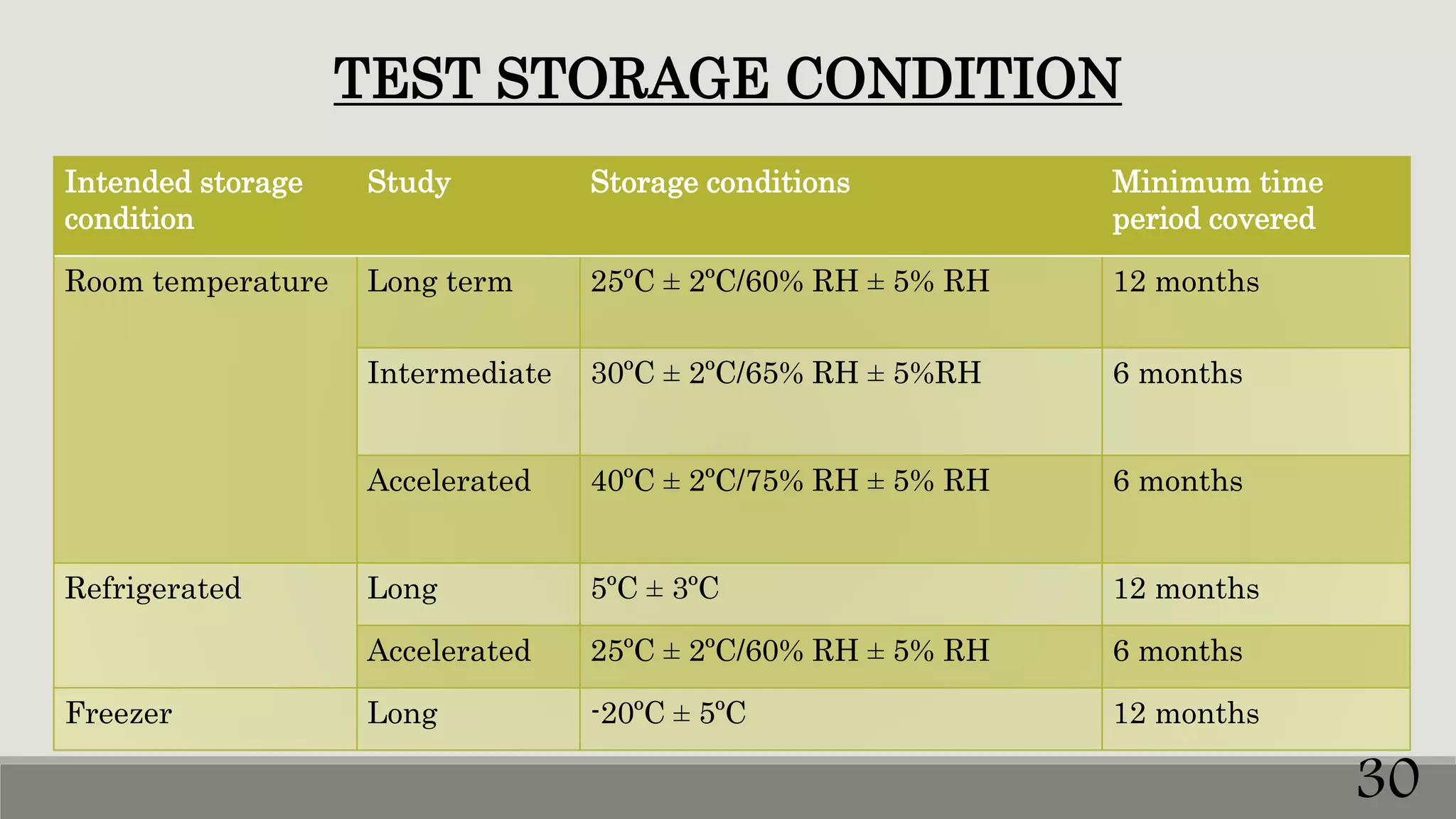
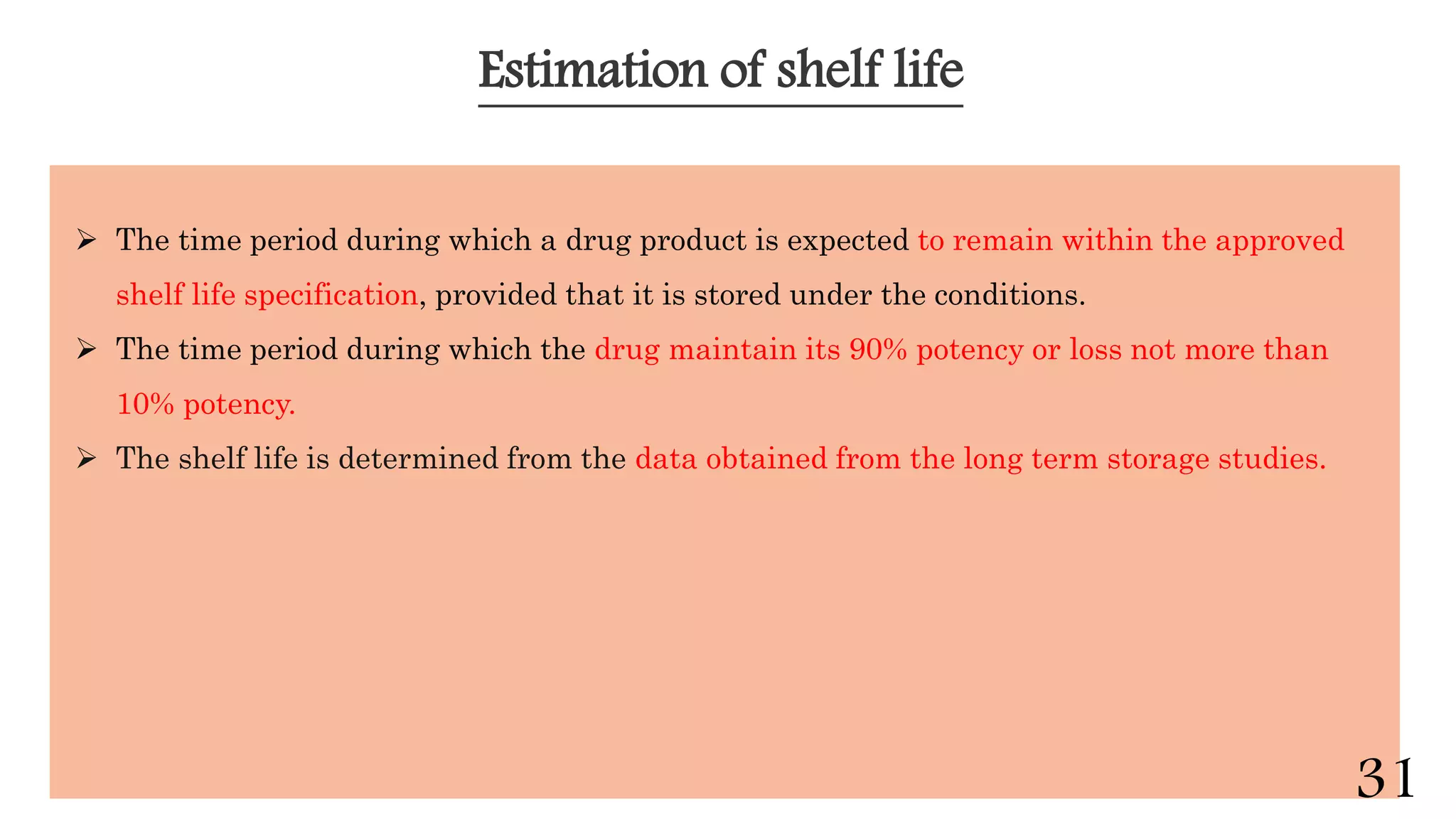
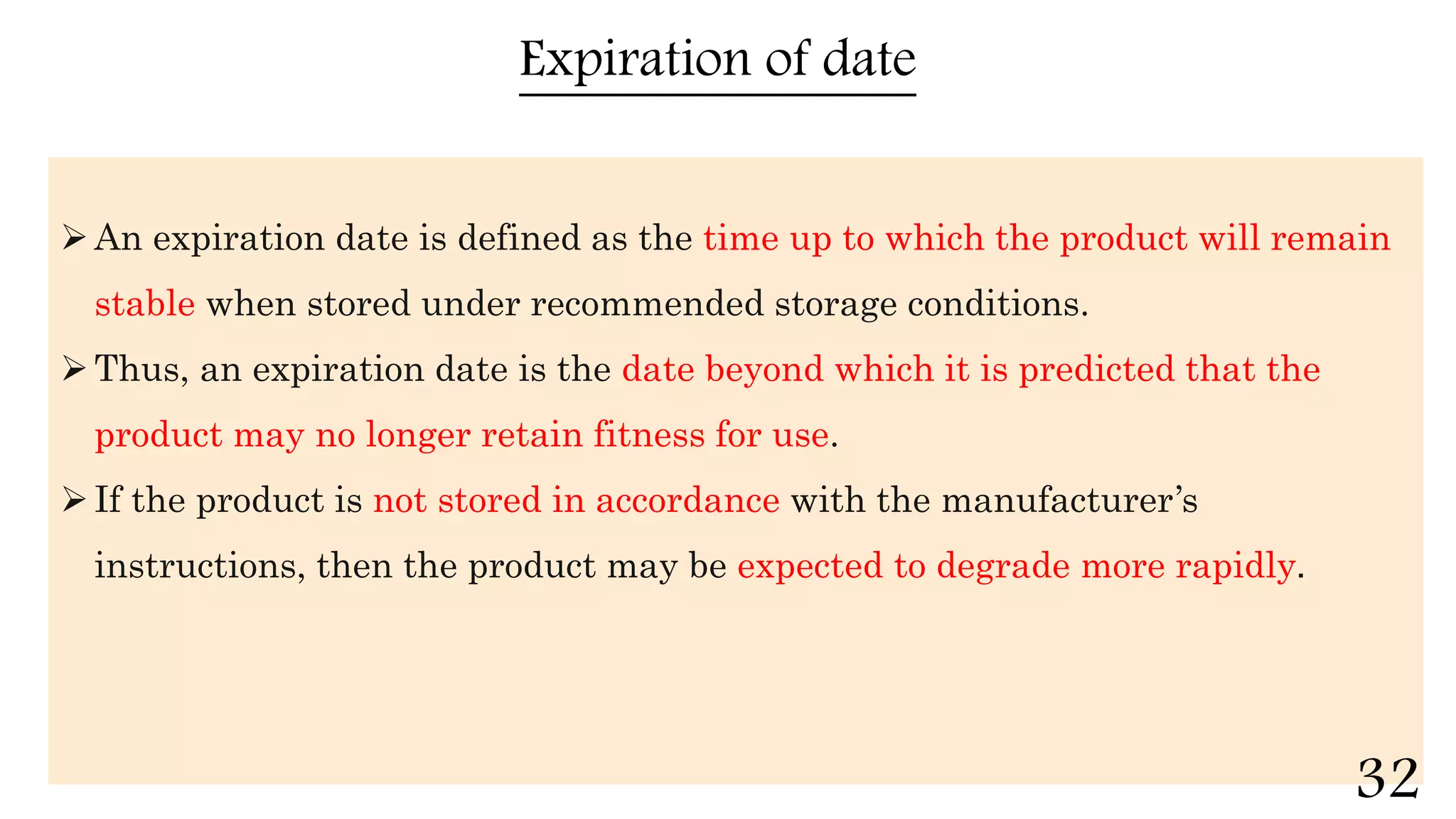
This document discusses kinetics of stability and stability testing. It defines drug kinetics as how a drug changes over time and explains zero and first order reaction kinetics. Factors affecting reaction rate and types of drug degradation are covered. Stability testing is defined and its importance, types, methods, guidelines and climatic zones are summarized. Methods for estimating shelf life and determining expiration dates are also presented.