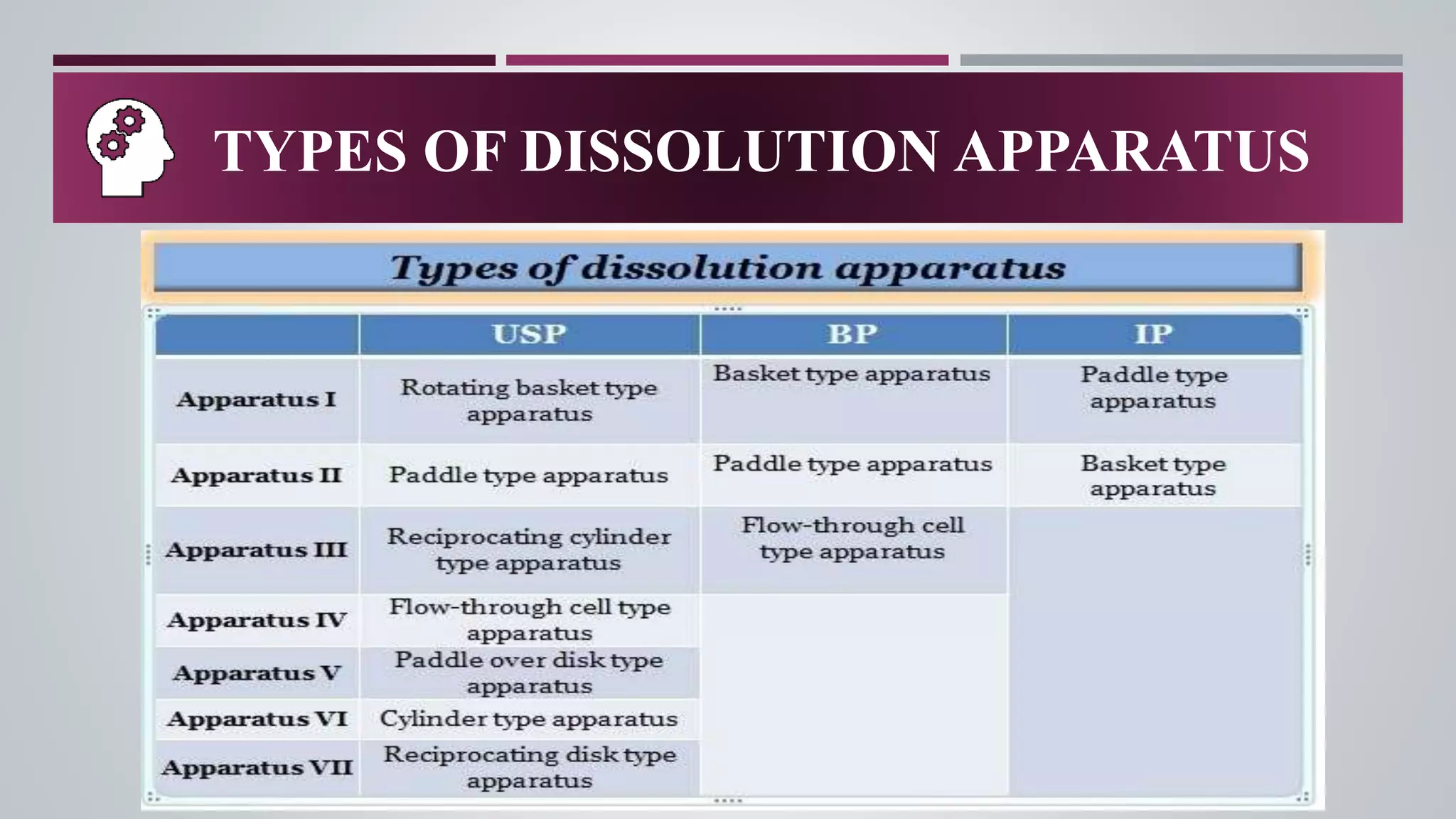
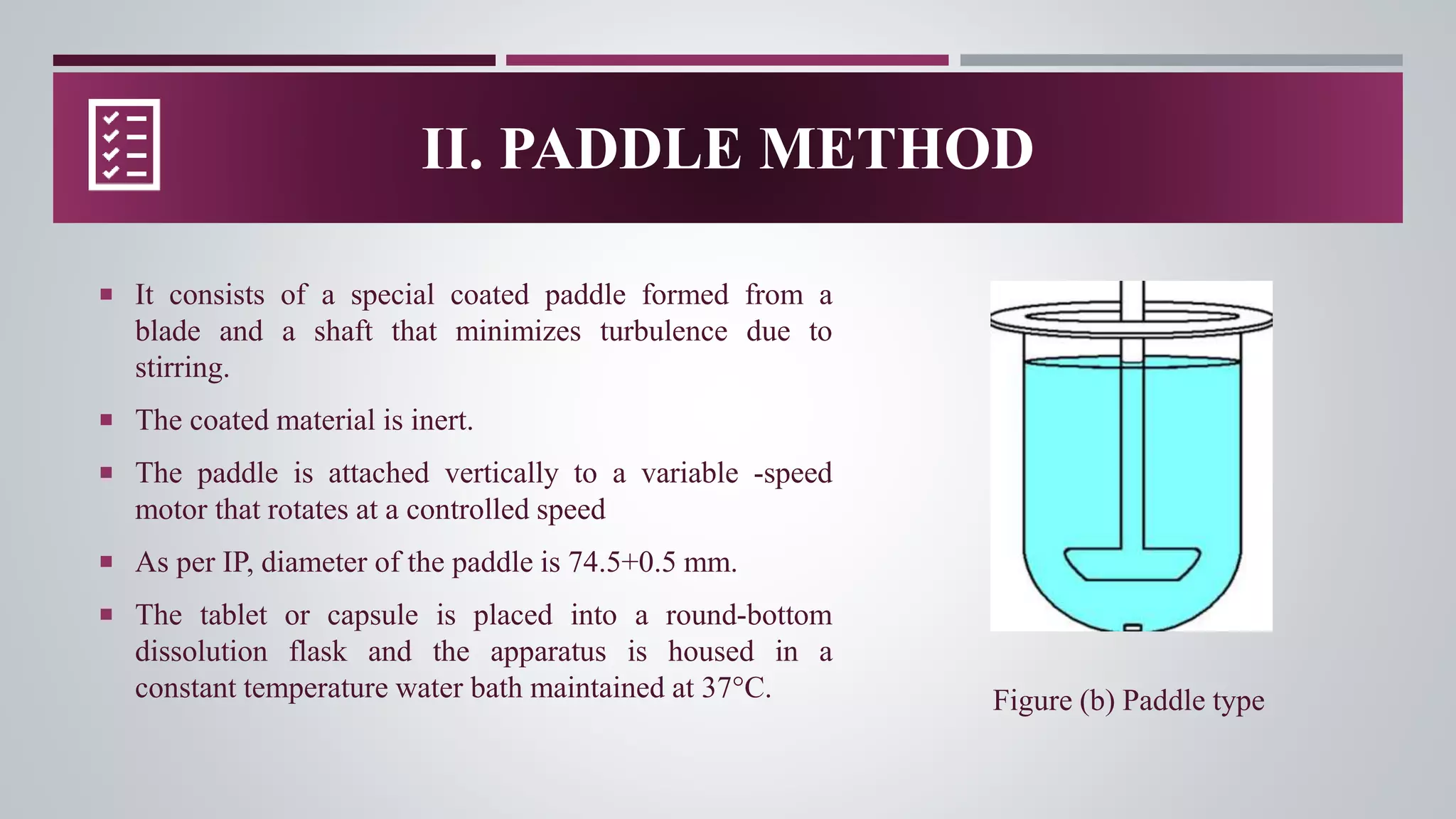
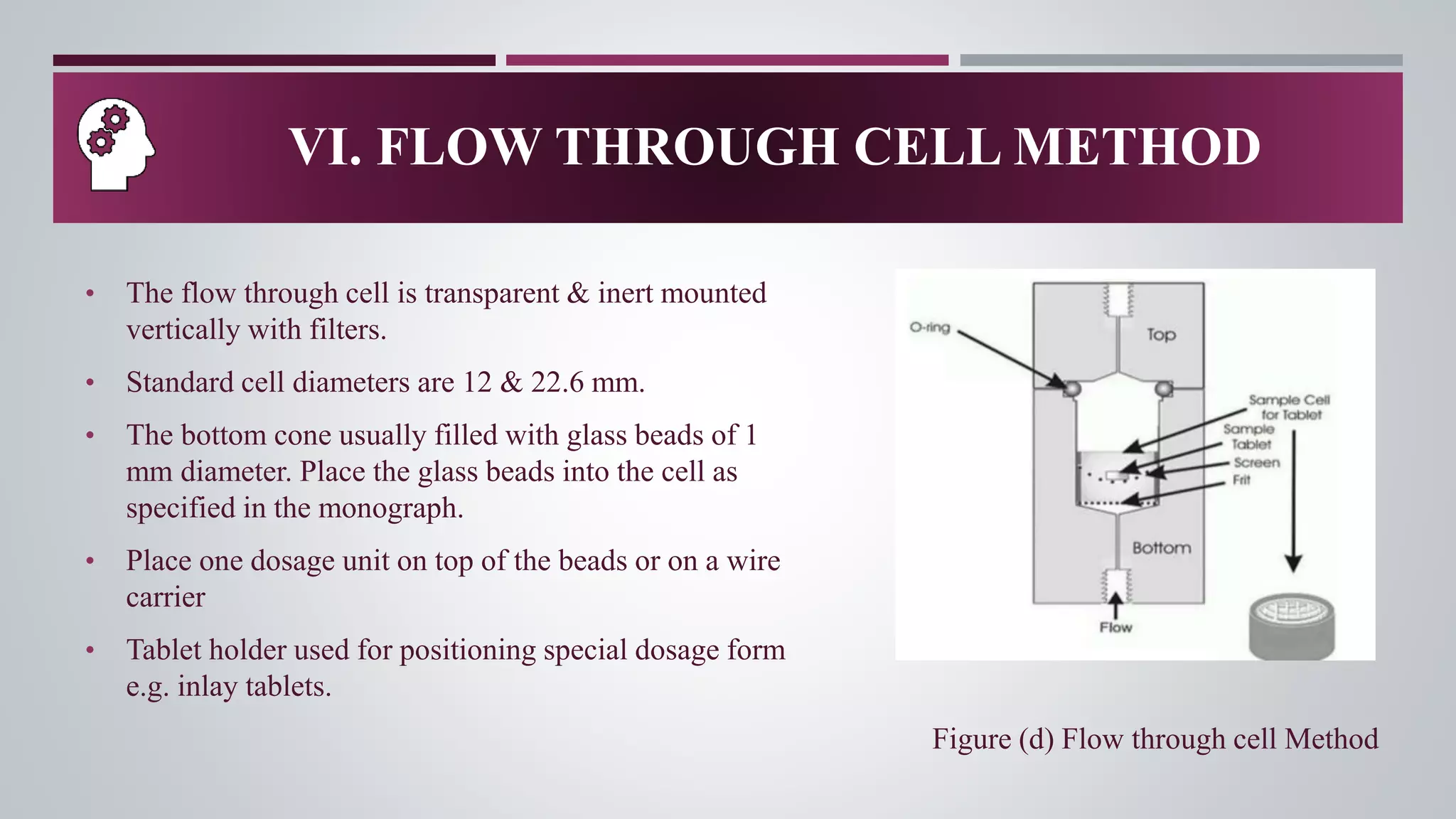
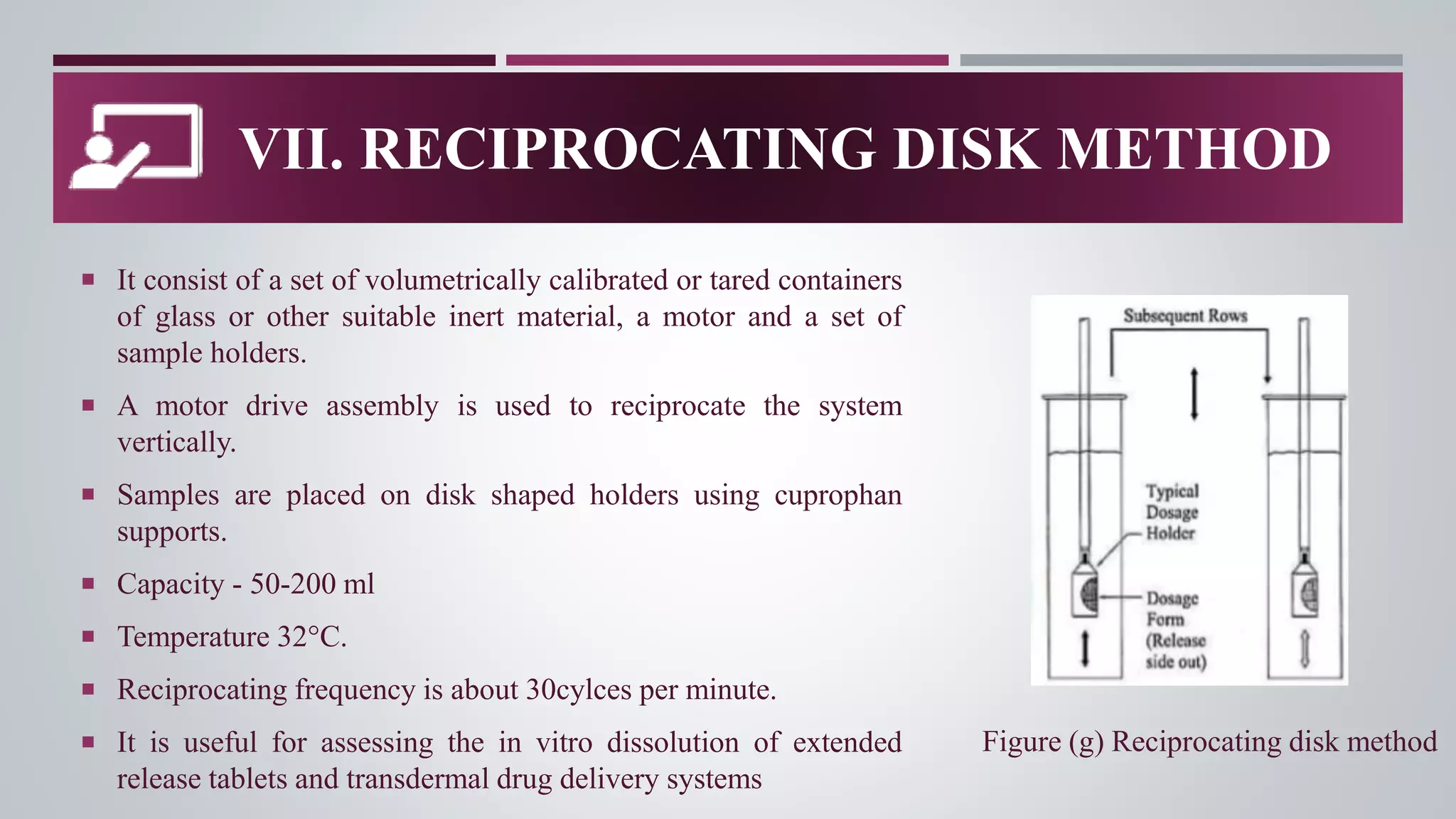
In-vitro dissolution testing is used to obtain information about the performance of drug products as they dissolve. There are several types of dissolution apparatus specified by the USP and IP that use different mechanisms like baskets, paddles, cylinders or flow-through cells to test dissolution under controlled temperature and flow conditions. The most common types are the rotating basket apparatus and paddle apparatus, which rotate or stir dosage forms like tablets in a dissolution medium maintained at 37°C to assess the rate and extent of drug release over time.