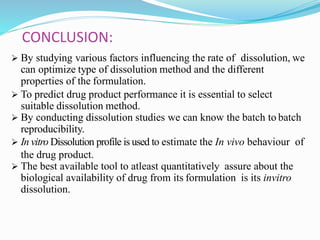
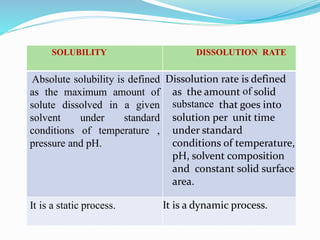
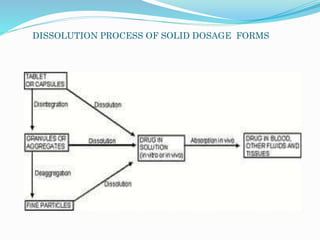
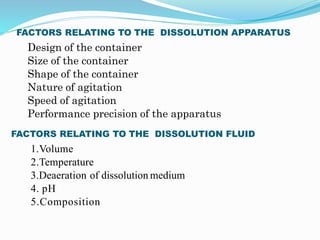
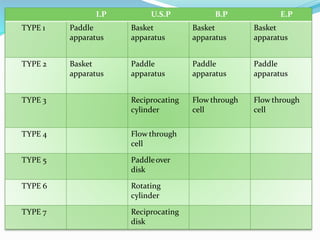
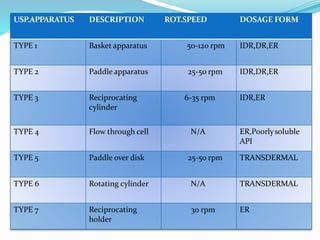
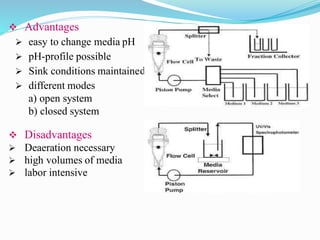
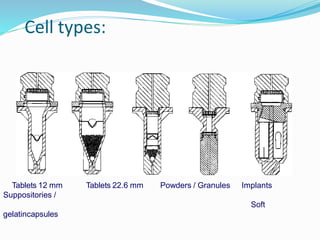
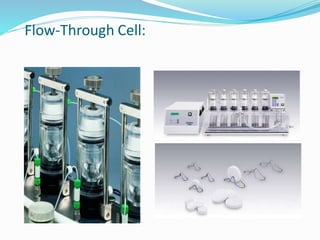
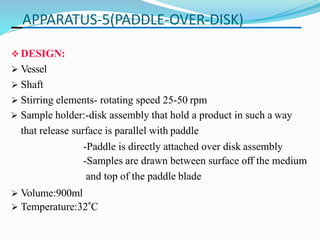
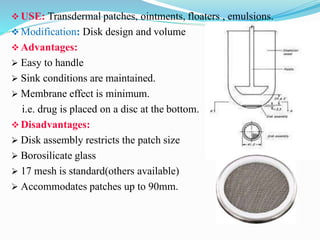
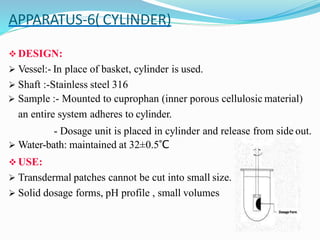
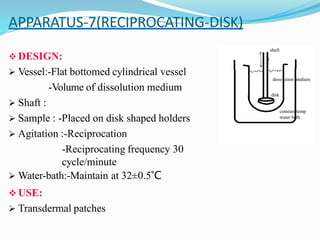
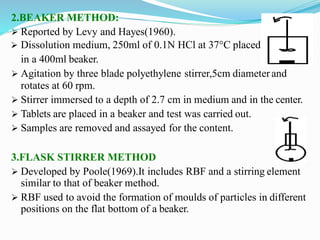
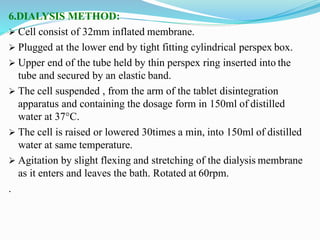
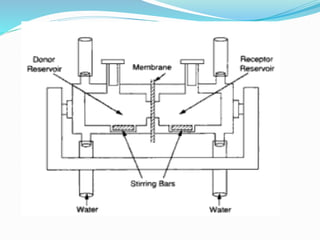
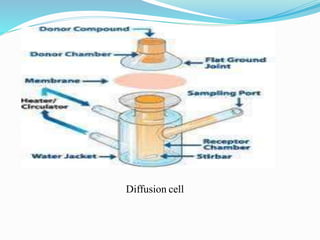
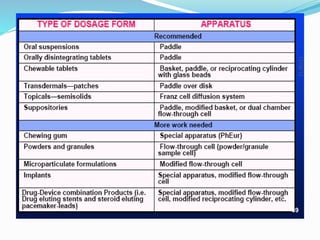
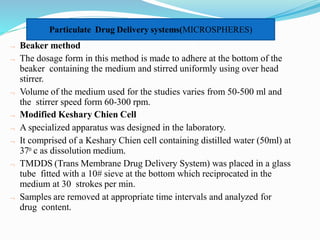
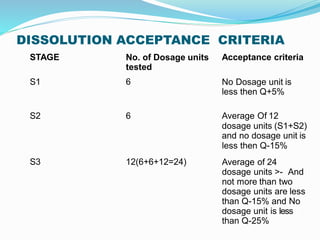
This document discusses in vitro dissolution testing methods. It defines dissolution as the process by which a solid substance solubilizes in a solvent, and dissolution rate as the amount of drug substance that goes into solution per unit time under standardized conditions. It then describes 7 common apparatus used for in vitro dissolution testing according to pharmacopeial standards, including the rotating basket, paddle, reciprocating cylinder, flow through cell, paddle over disk, rotating cylinder, and reciprocating disk methods. Each apparatus has distinct advantages and disadvantages for testing different drug products and dosage forms.




























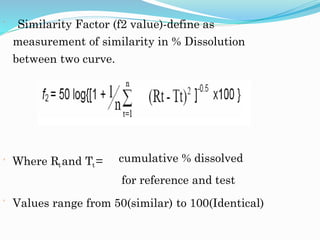
![COMPARISON OF DISSOLUTION PROFILE
Difference factor (f1 Value)-
Define as calculate the % Difference between 2
curves at each time point and is a measurement of
the relative error between 2 curves.
f1= {[Σ t=1n |Rt-Tt|] / [Σ t=1n Rt]} ×100.
Values range from 0 to 15
47](https://image.slidesharecdn.com/invitrodissolutiontestingmethods-200528071550/85/In-vitro-dissolution-testing-methods-58-320.jpg)