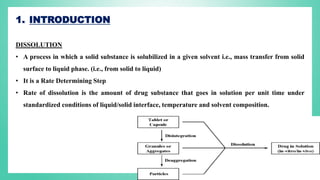
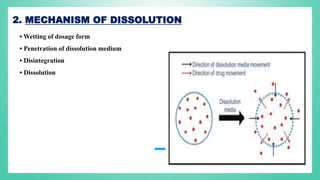
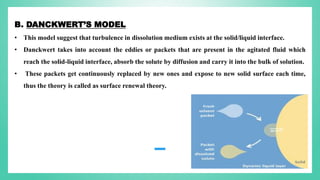
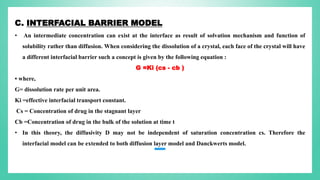
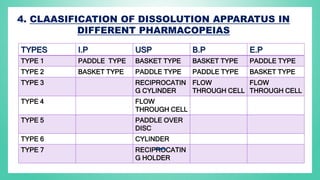
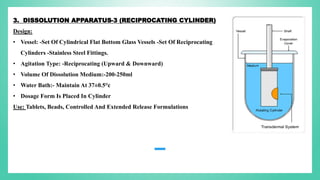
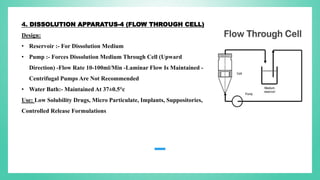
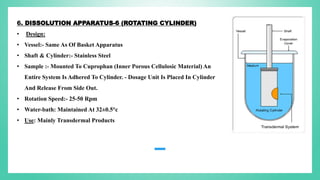
The document outlines the process of dissolution, including mechanisms, models, classifications of apparatus, and factors affecting dissolution rates. It explains various theories such as the diffusion layer model and Danckwert’s model, and presents different types of dissolution apparatus used in pharmaceuticals. Additionally, it discusses the problems of variable control in dissolution testing performance which can arise from equipment, process, and drug product properties.