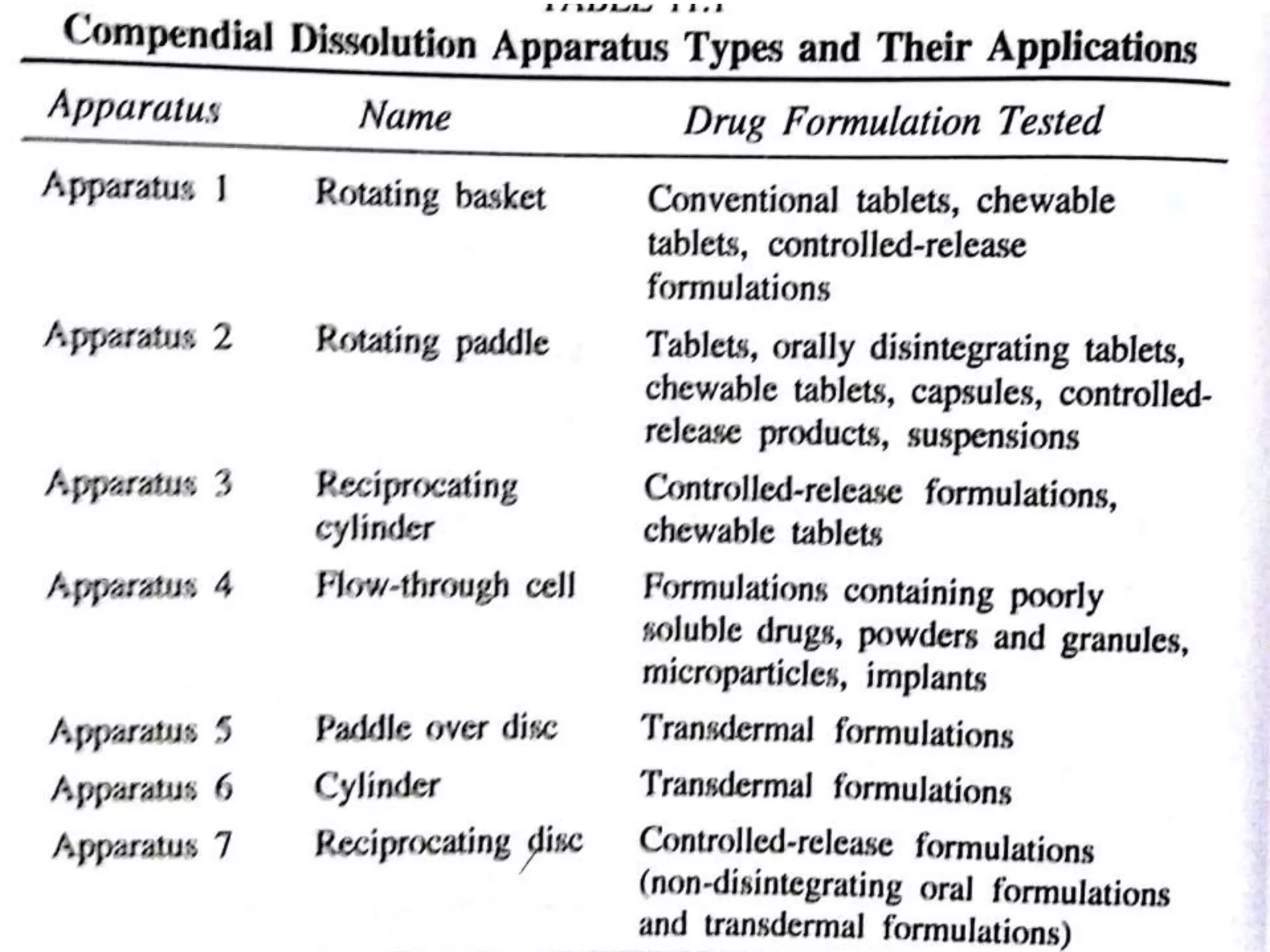
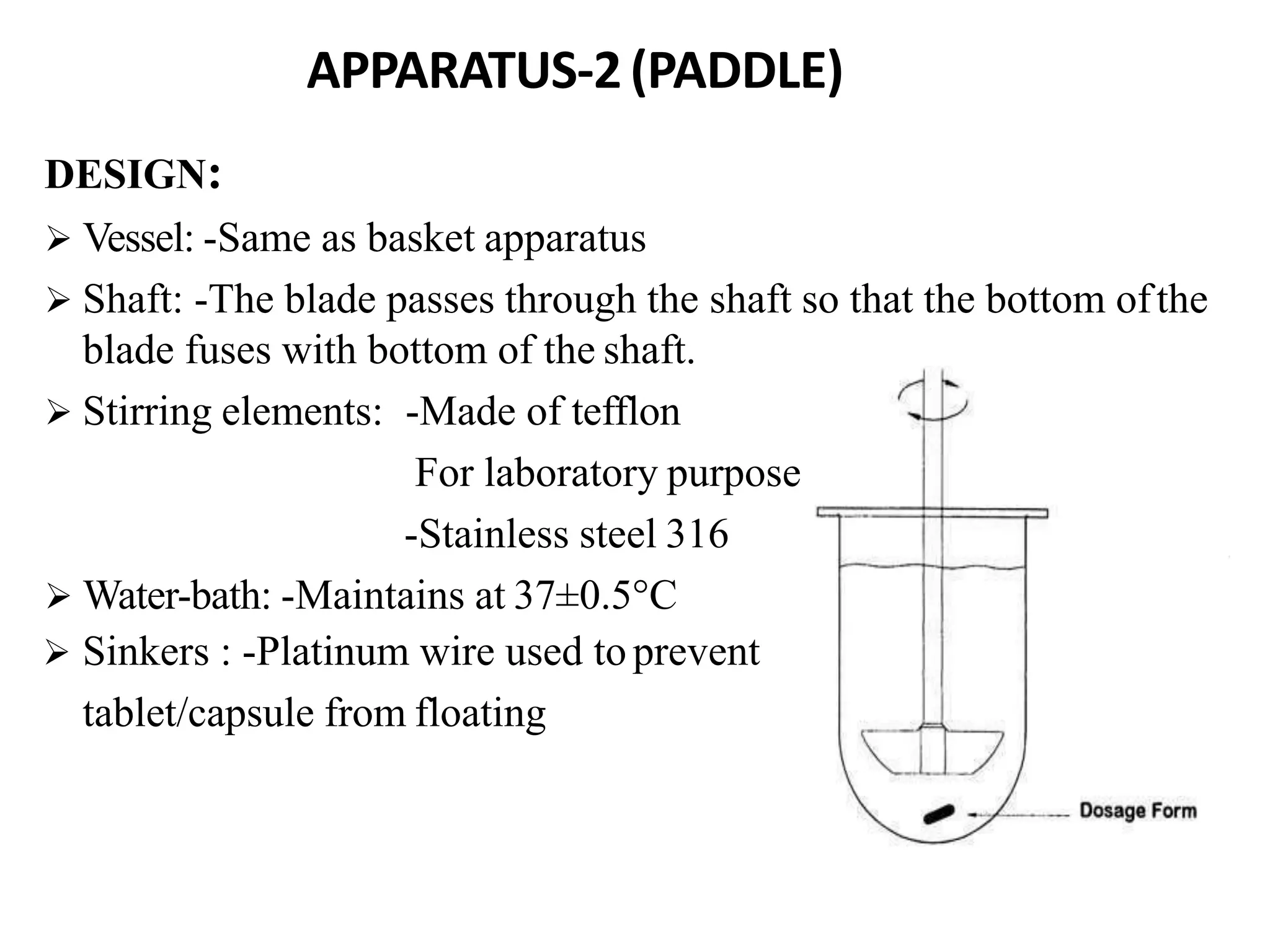
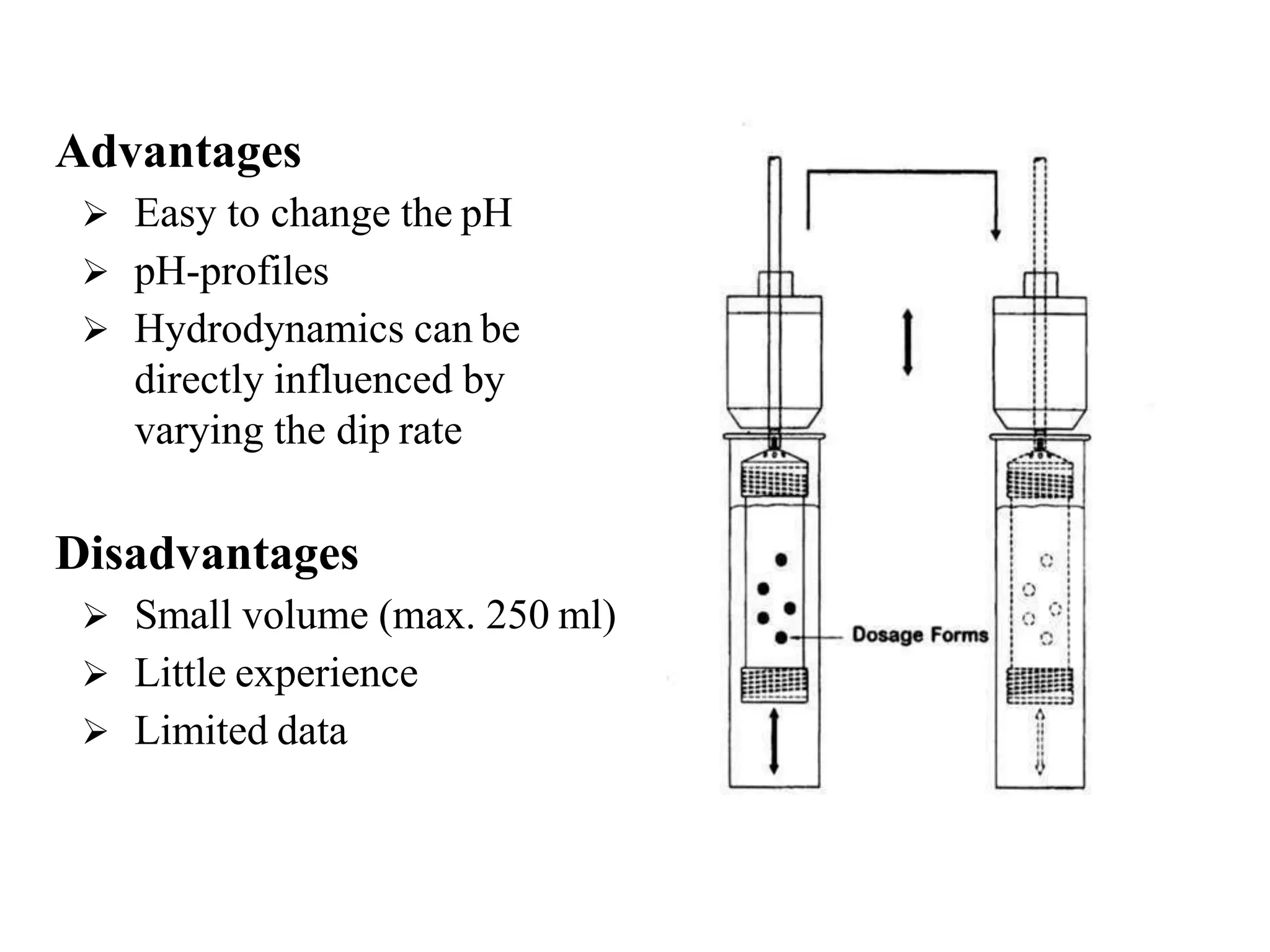
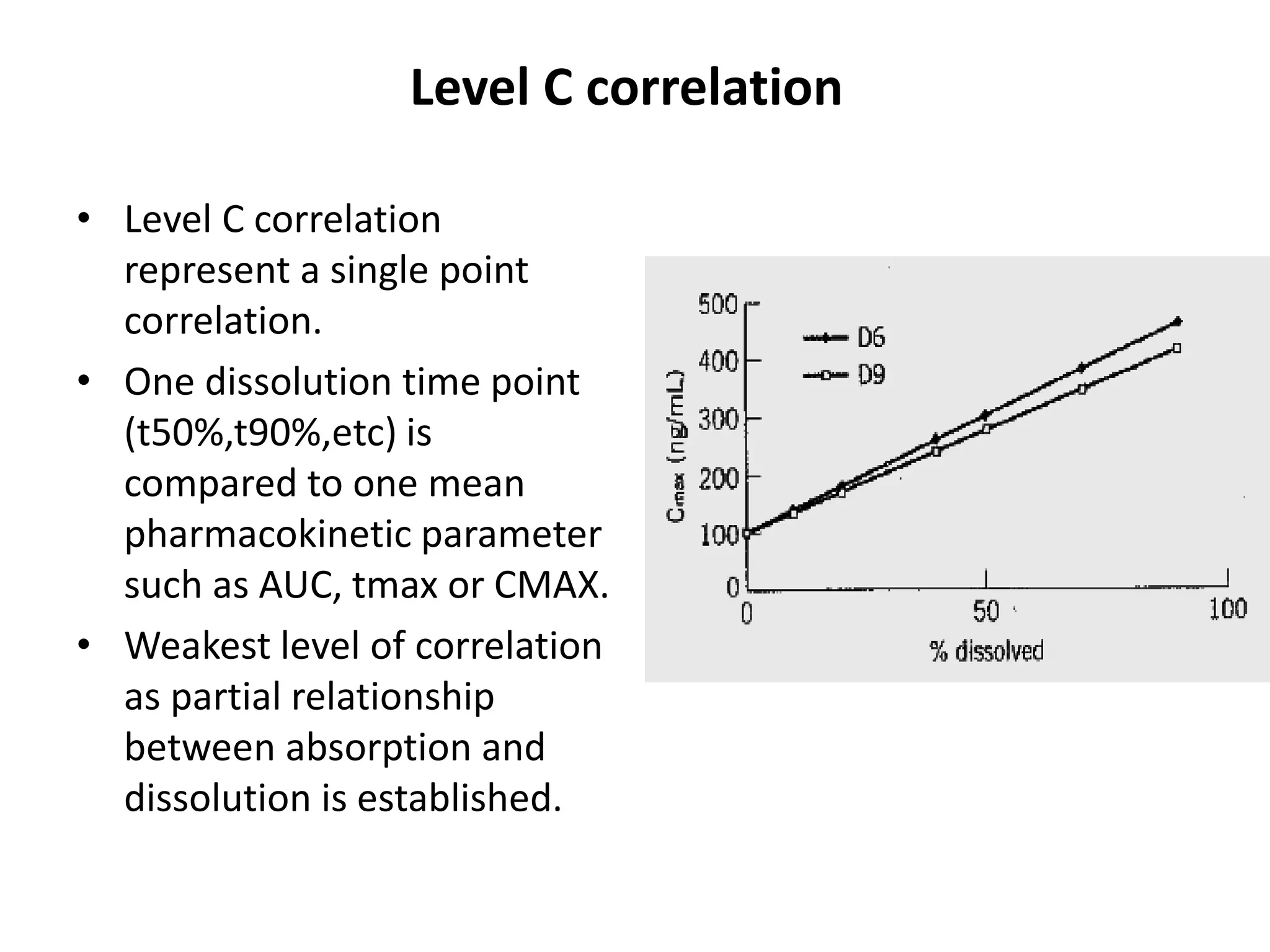
The document summarizes a seminar presentation on in vitro dissolution and in vitro-in vivo correlation (IVIVC). It defines key terms like dissolution, IVIVC, and discusses the significance of IVIVC including its use in reducing bioequivalence studies. The document also describes the various apparatus used for in vitro dissolution testing and the parameters and levels used to establish correlations between in vitro dissolution and in vivo absorption.