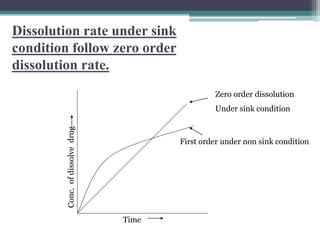
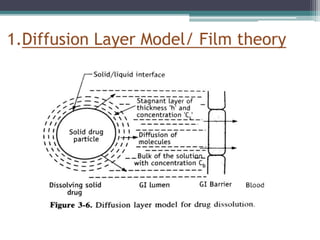
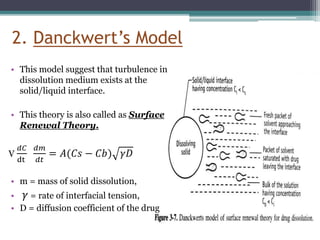
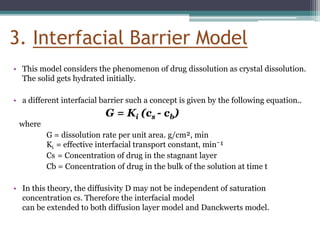
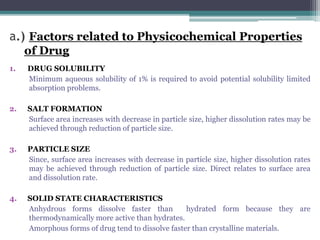
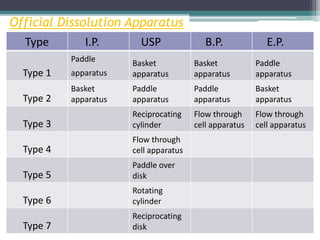
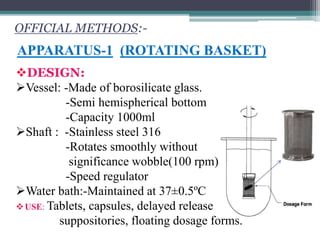
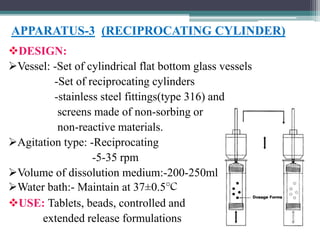
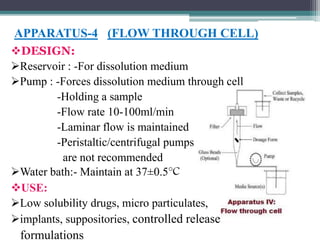
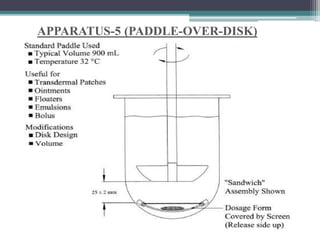
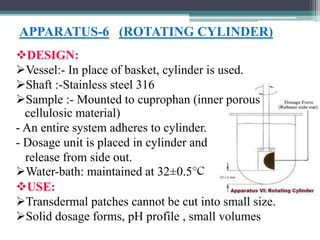
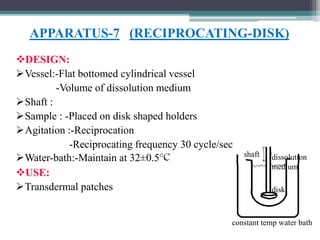
The document discusses dissolution models and methods. It defines dissolution as the process by which a solid substance is solubilized in a solvent. Several theories of dissolution are described, including the diffusion layer model, Danckwert's model, and the interfacial barrier model. Factors that can affect dissolution, such as drug properties, formulation components, and test conditions are outlined. Both official and unofficial in vitro dissolution test models are reviewed, including common apparatus like the paddle and basket methods. Finally, the kinetics of dissolution are discussed and equations for modeling zero-order, first-order, Higuchi, Hixson-Crowell, and Korsmeyer-Peppas release are provided.










![•
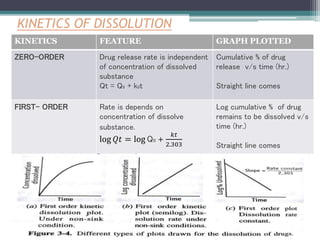
KINETICS FEATURE GRAPH PLOTTED
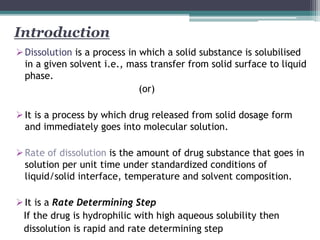
HIGUCHI- MODEL
Describes the release
by dissolution and
change in surface area
and diameter of
dissolved particles.
Q = KH t½
Initial concentration -
%drug remaining v/s
time (hr.)
Straight line comes
HIXON-CROWEL Its suggest drug release
by diffusion
mechanism.
𝐾ℎ𝑐𝑡 = 𝑄₀1/3− 𝑄𝑡1/3
Cumulative % of drug
release v/s square root
of time (hr.)
Straight line comes
KORSMEYER-
PEPPAS
[Mt/M] = Kn t⁰ Log cumulative % of
drug remaining to be
dissolve v/s log time
(hr.)
Straight line comes](https://image.slidesharecdn.com/dissolutionmodelsandmethods3-200725143507/85/Dissolution-Models-and-Methods-Factors-and-Kinetics-28-320.jpg)