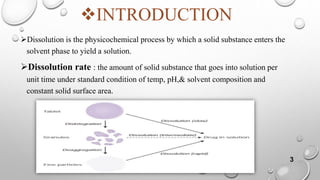
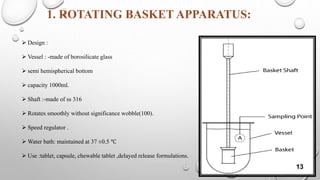
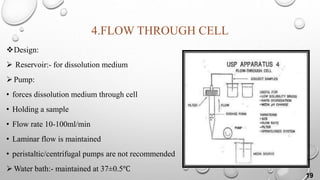
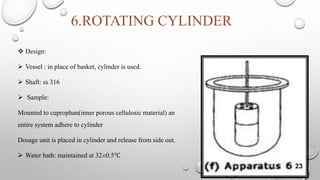
The document provides a comprehensive overview of dissolution testing, its importance in drug formulation, and various dissolution apparatuses used in pharmaceutical research. It explains the theories of drug dissolution, including the diffusion layer model, Danckwert's model, and the interfacial barrier model. The text concludes that dissolution testing is crucial for ensuring the quality and efficacy of pharmaceutical products.