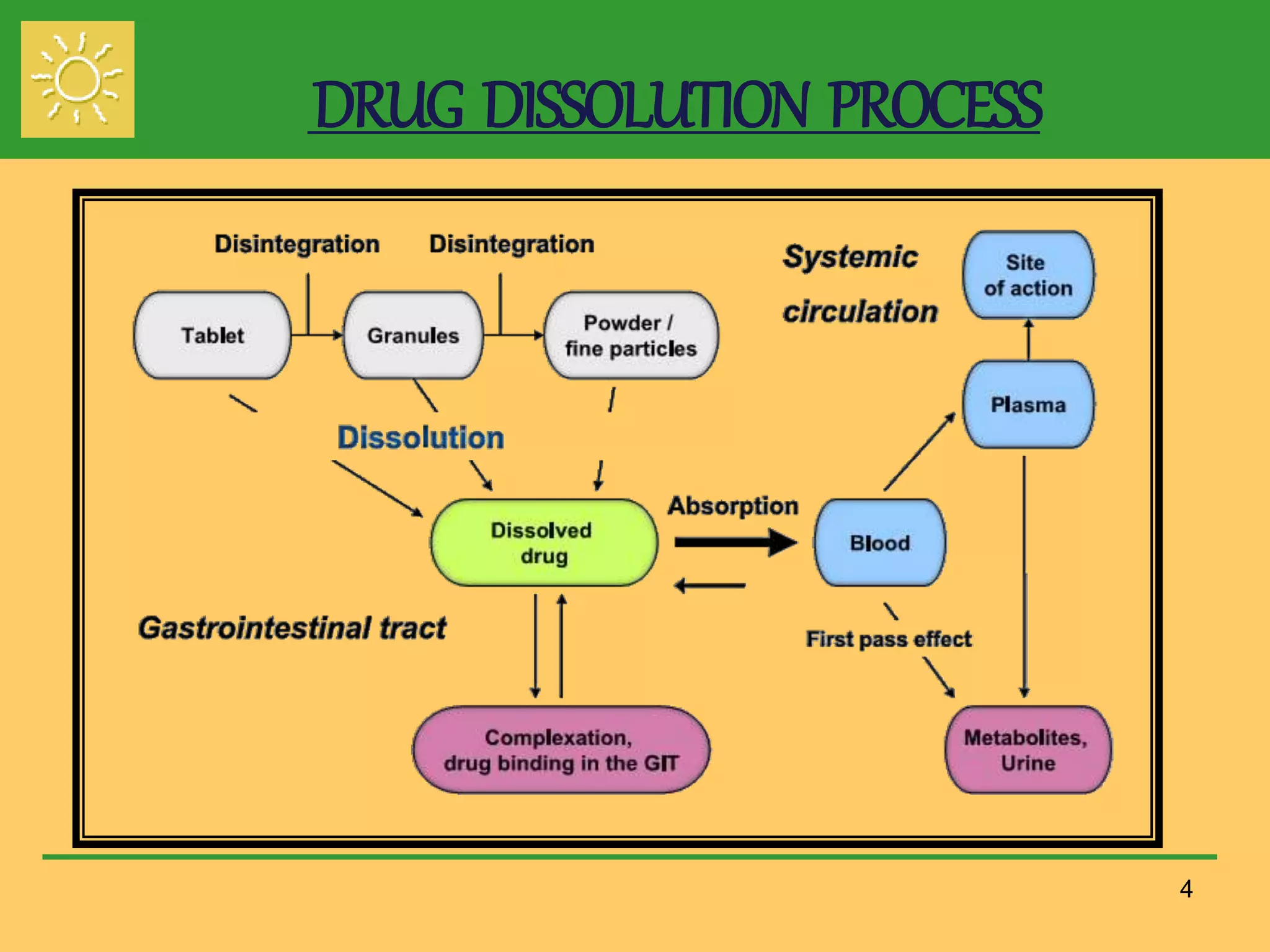
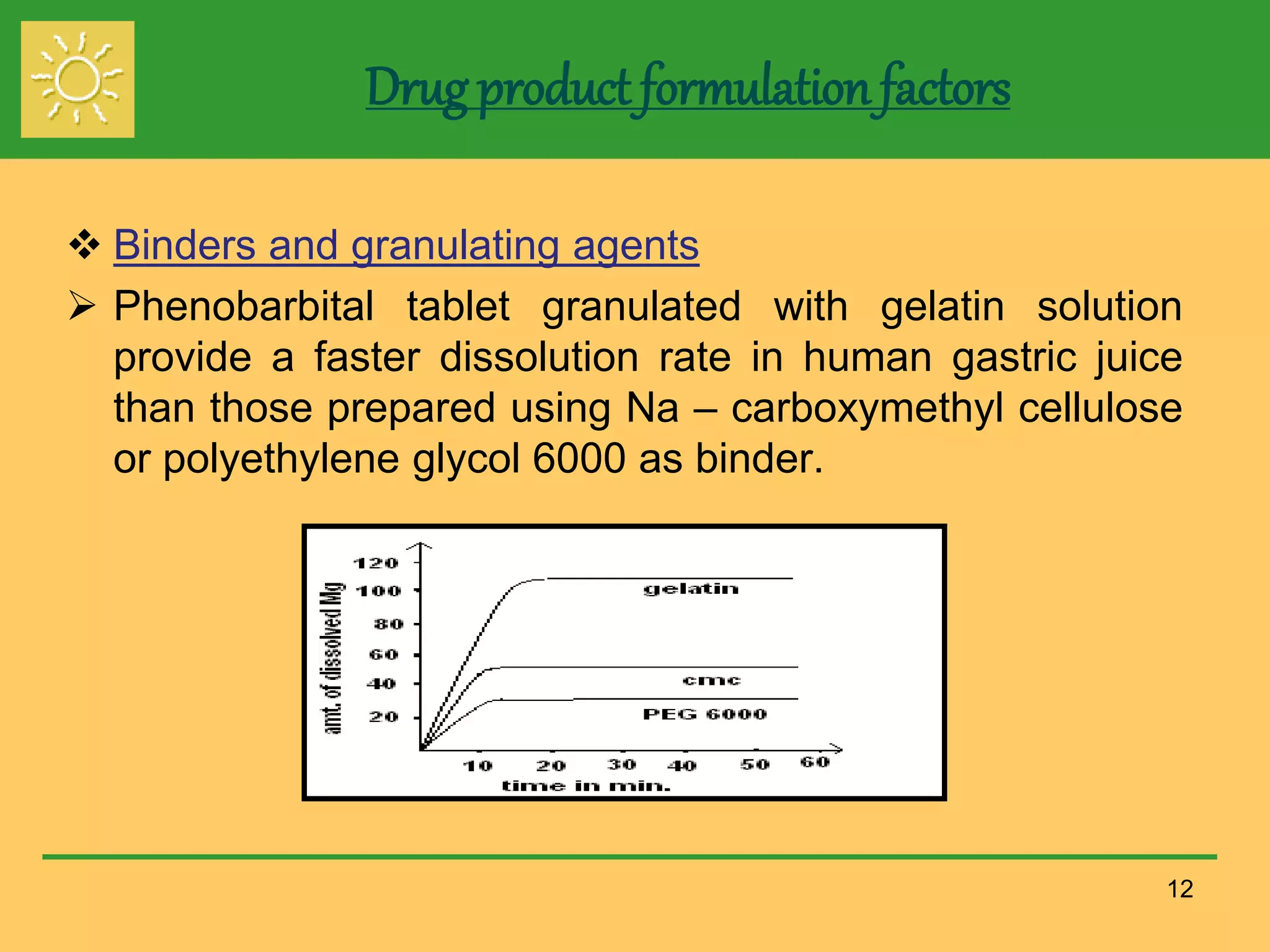
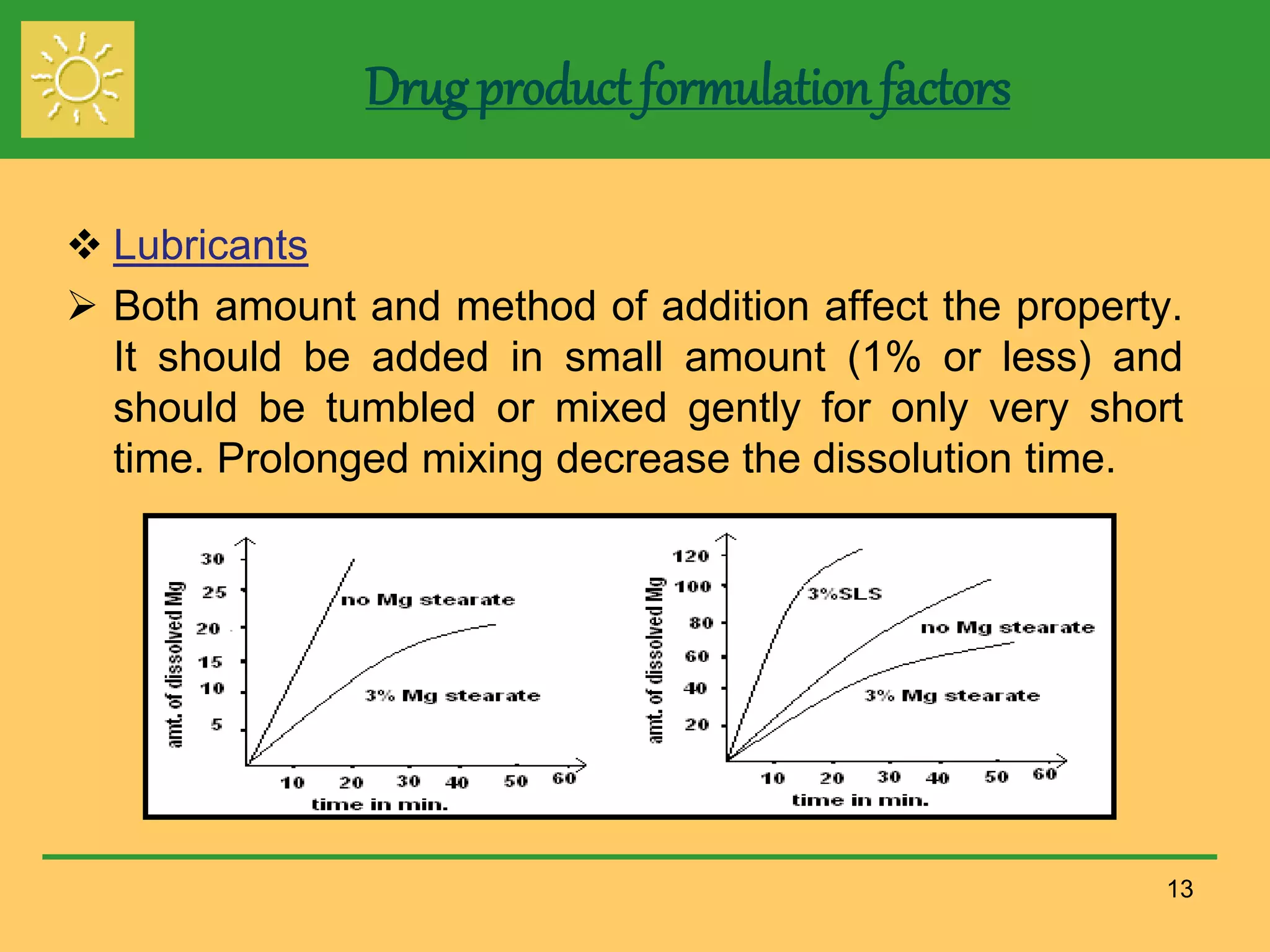
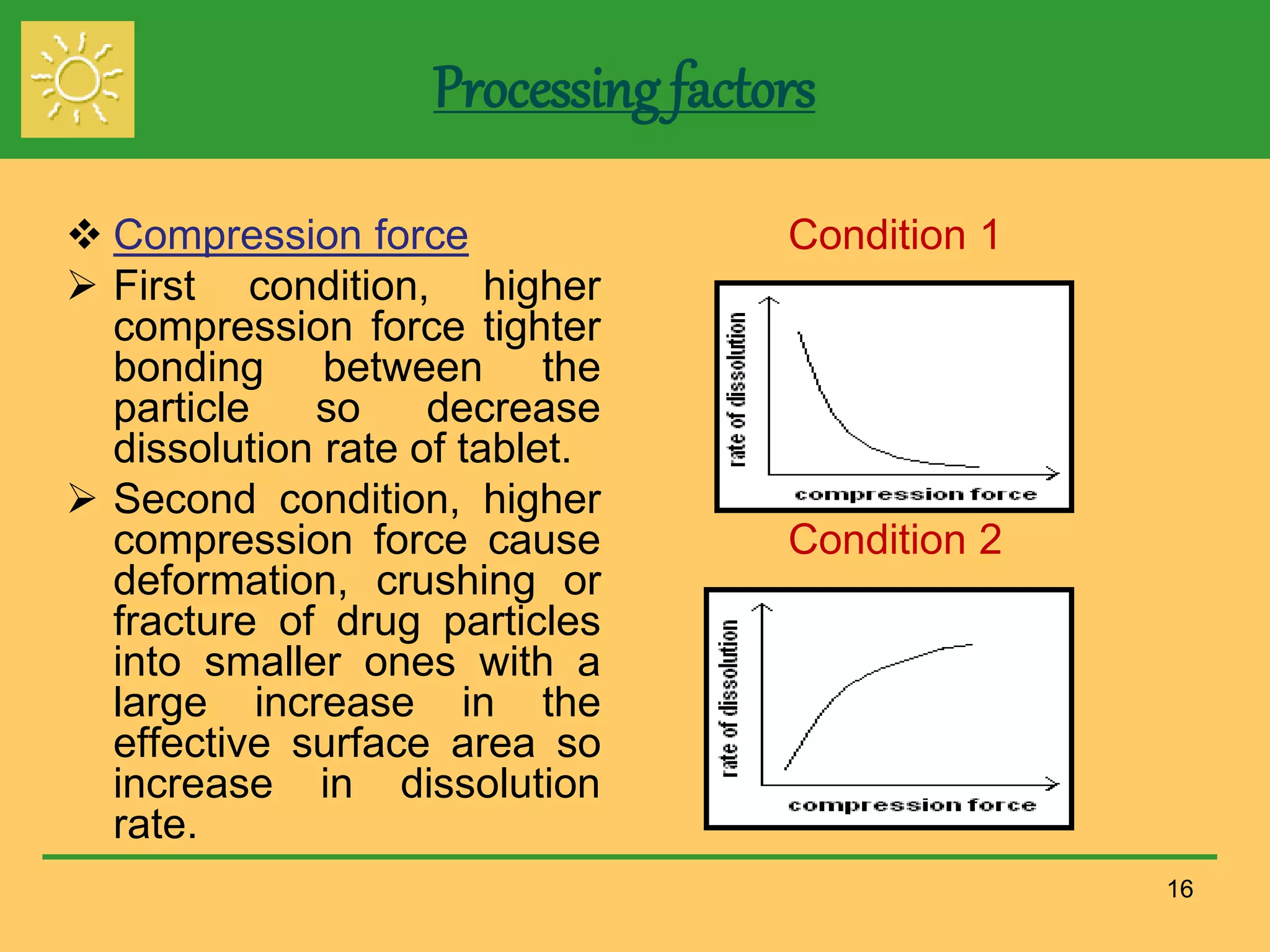
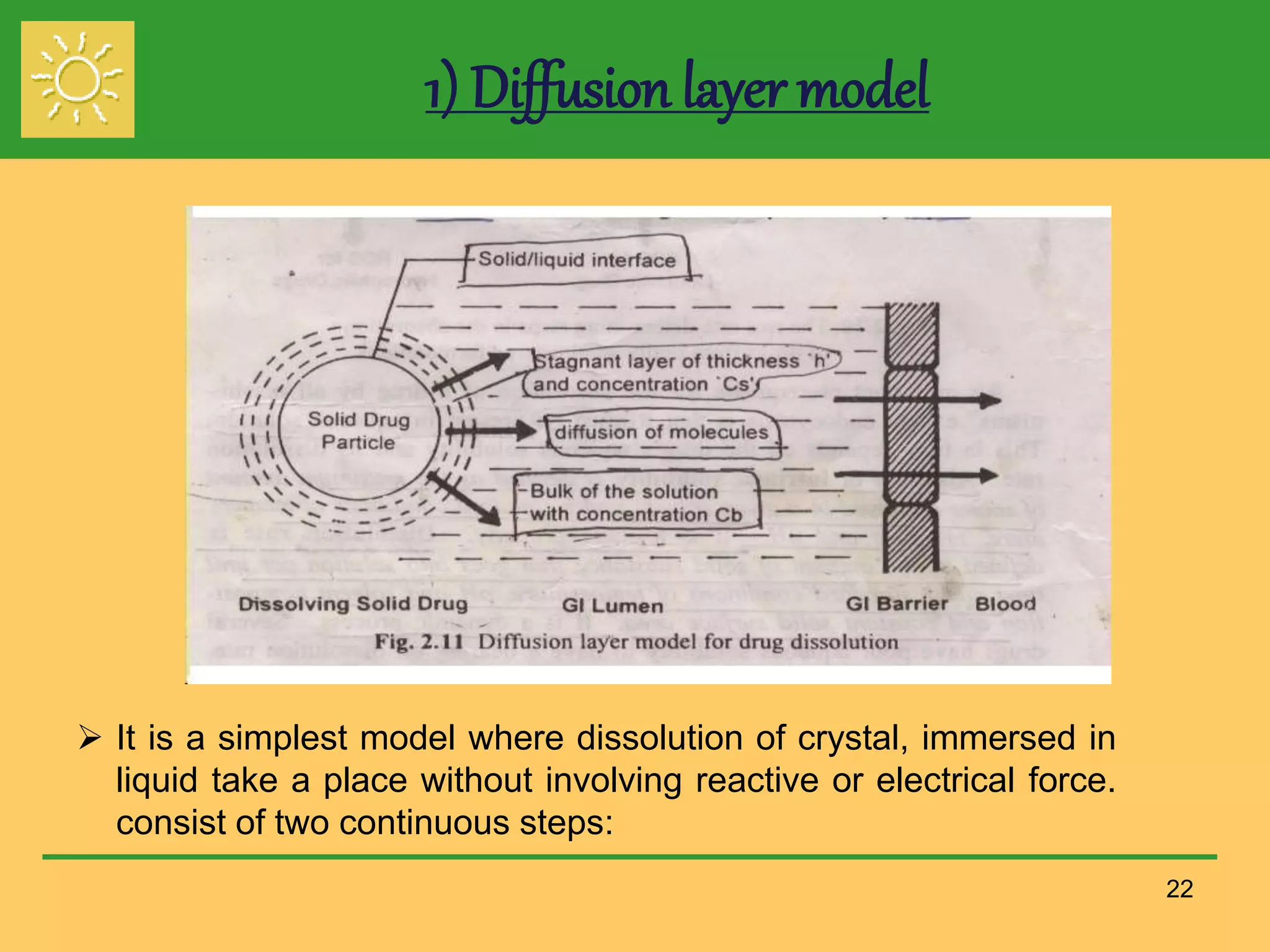
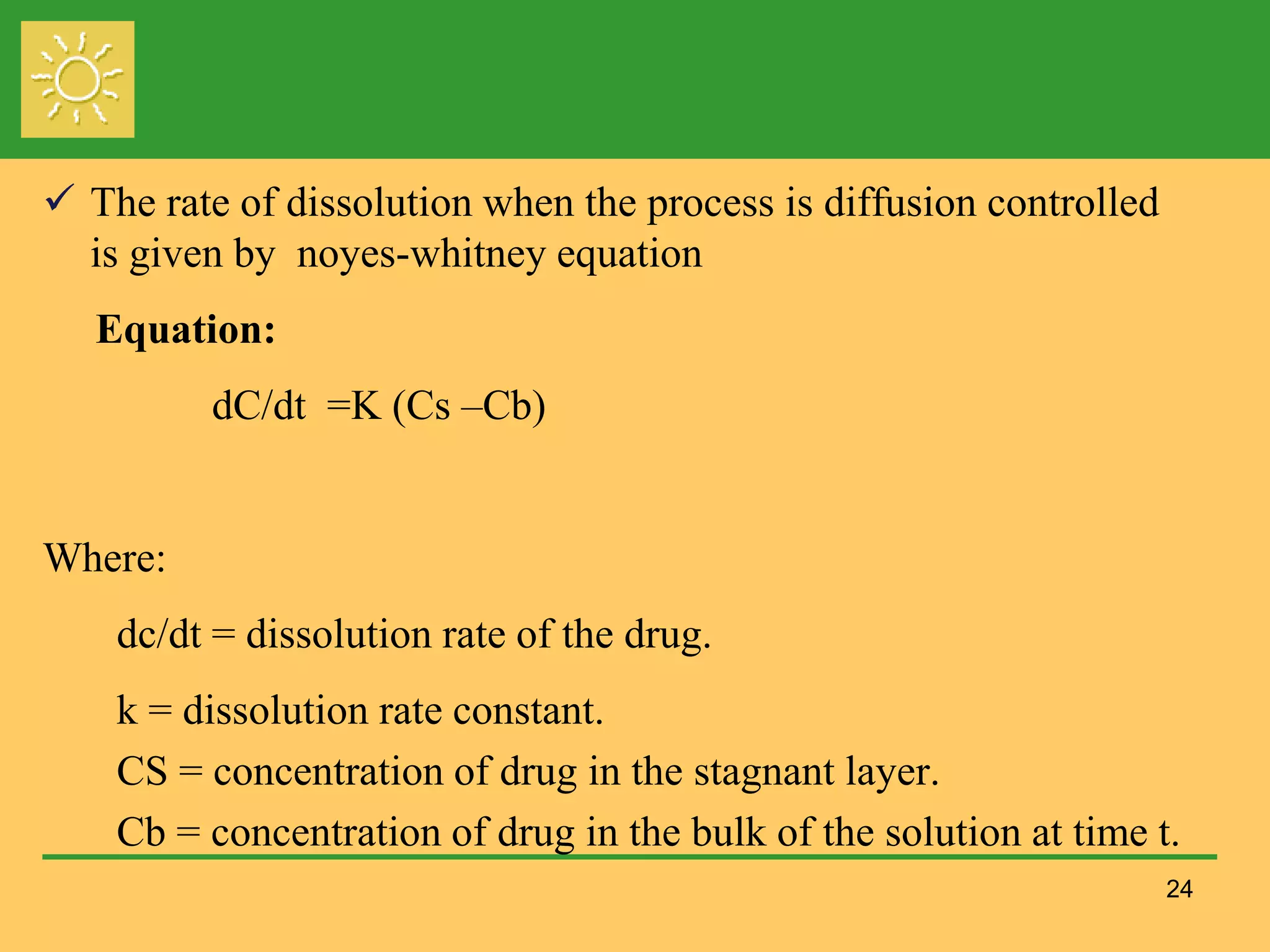
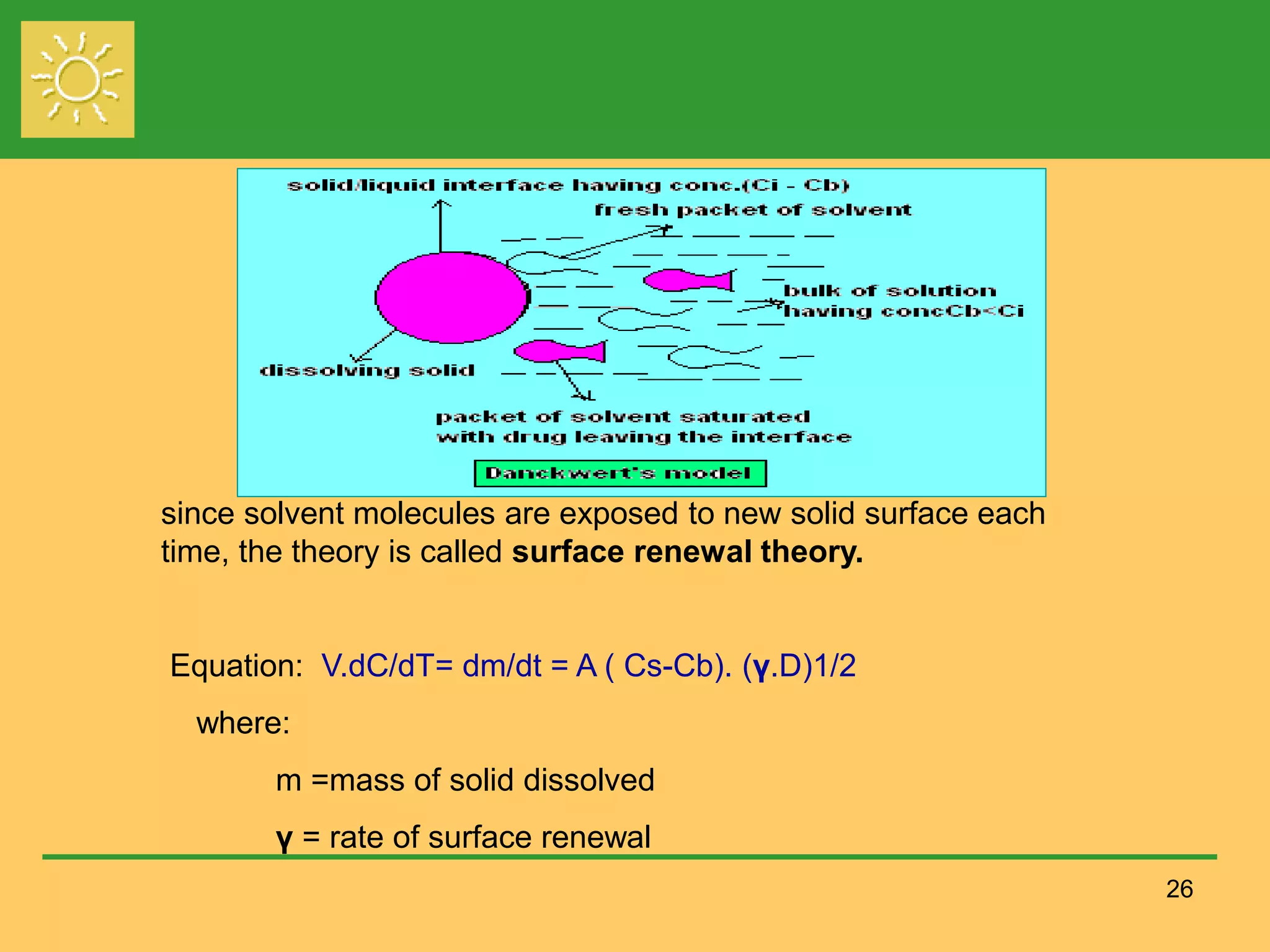
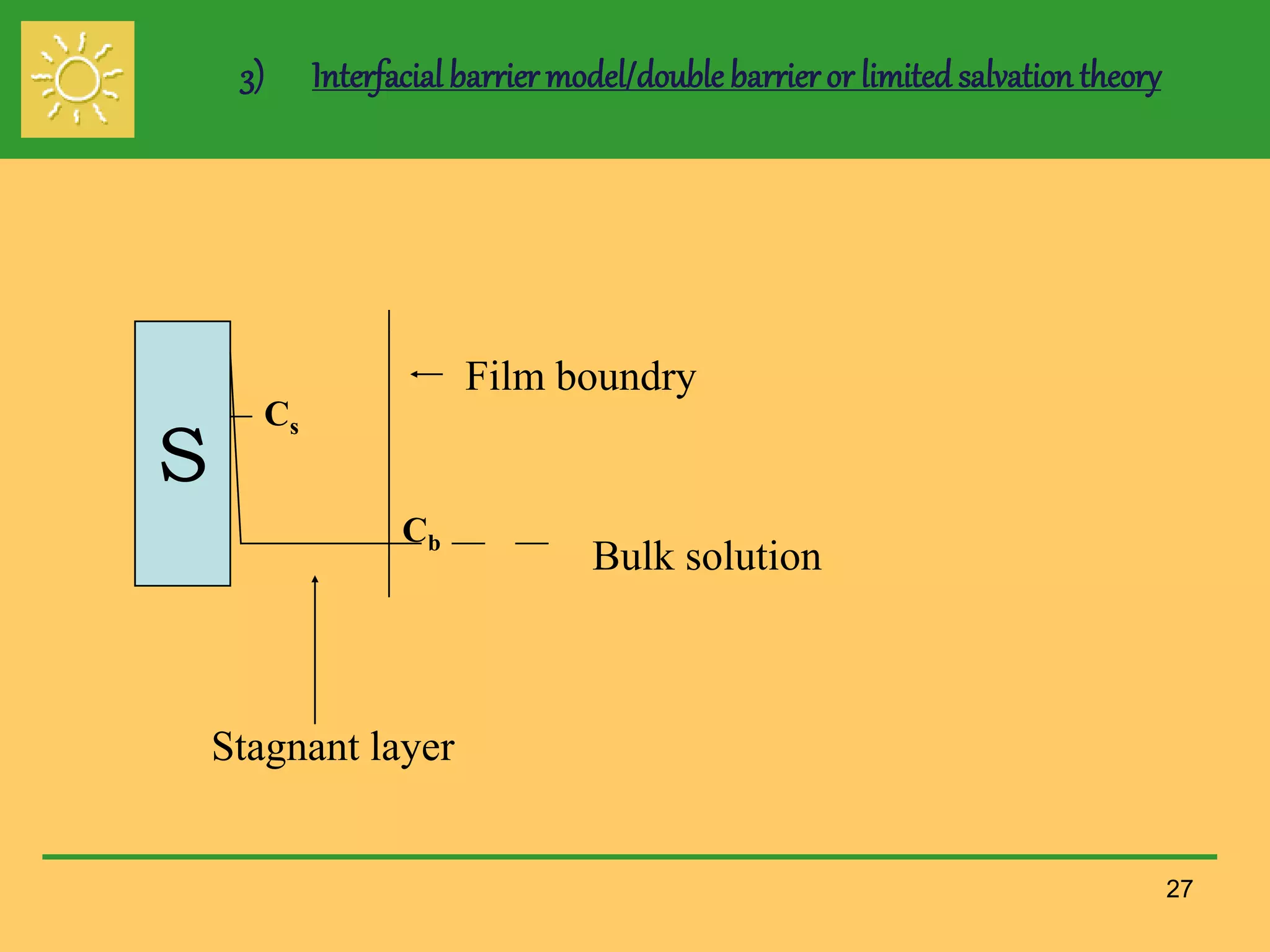
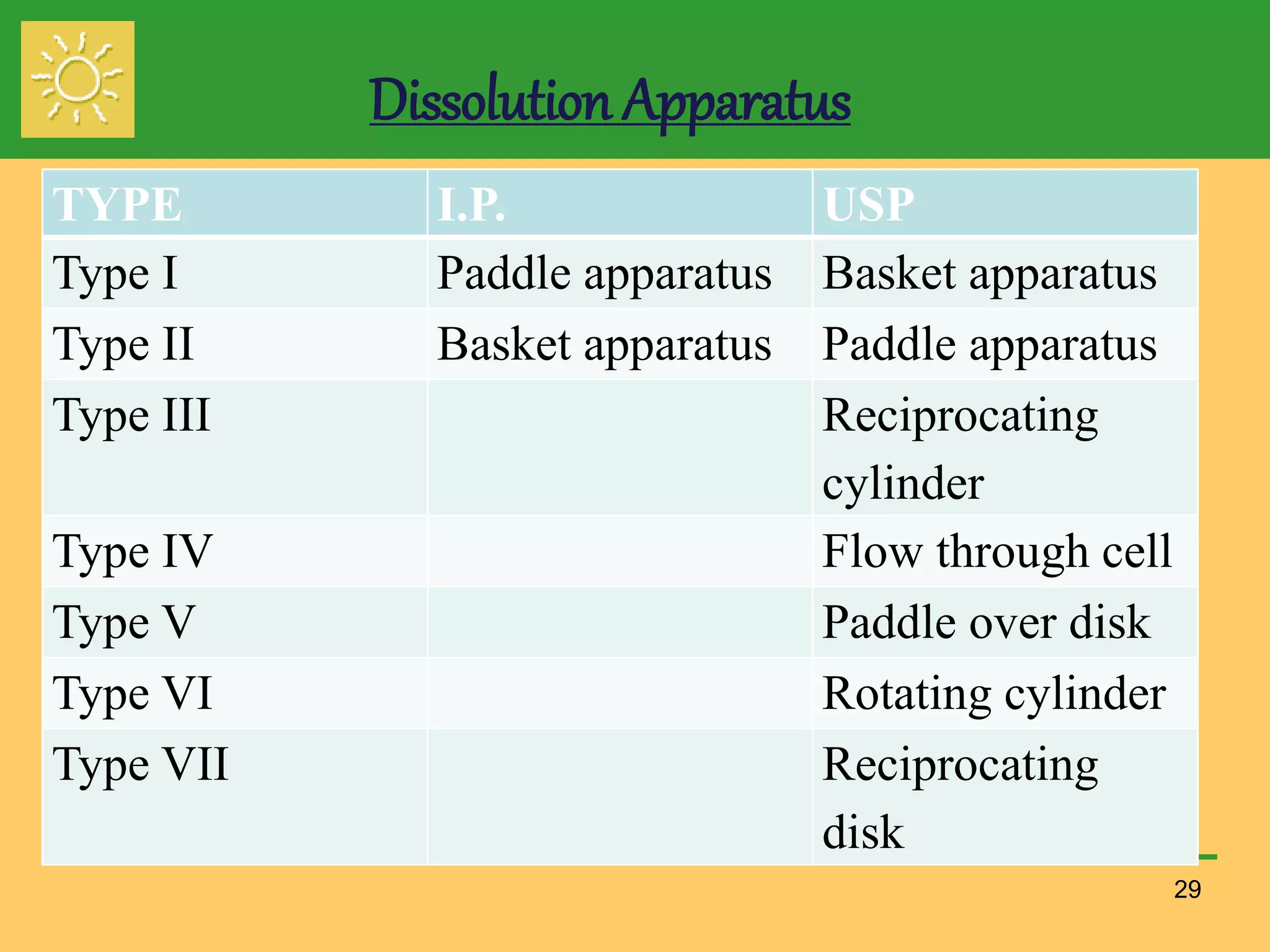
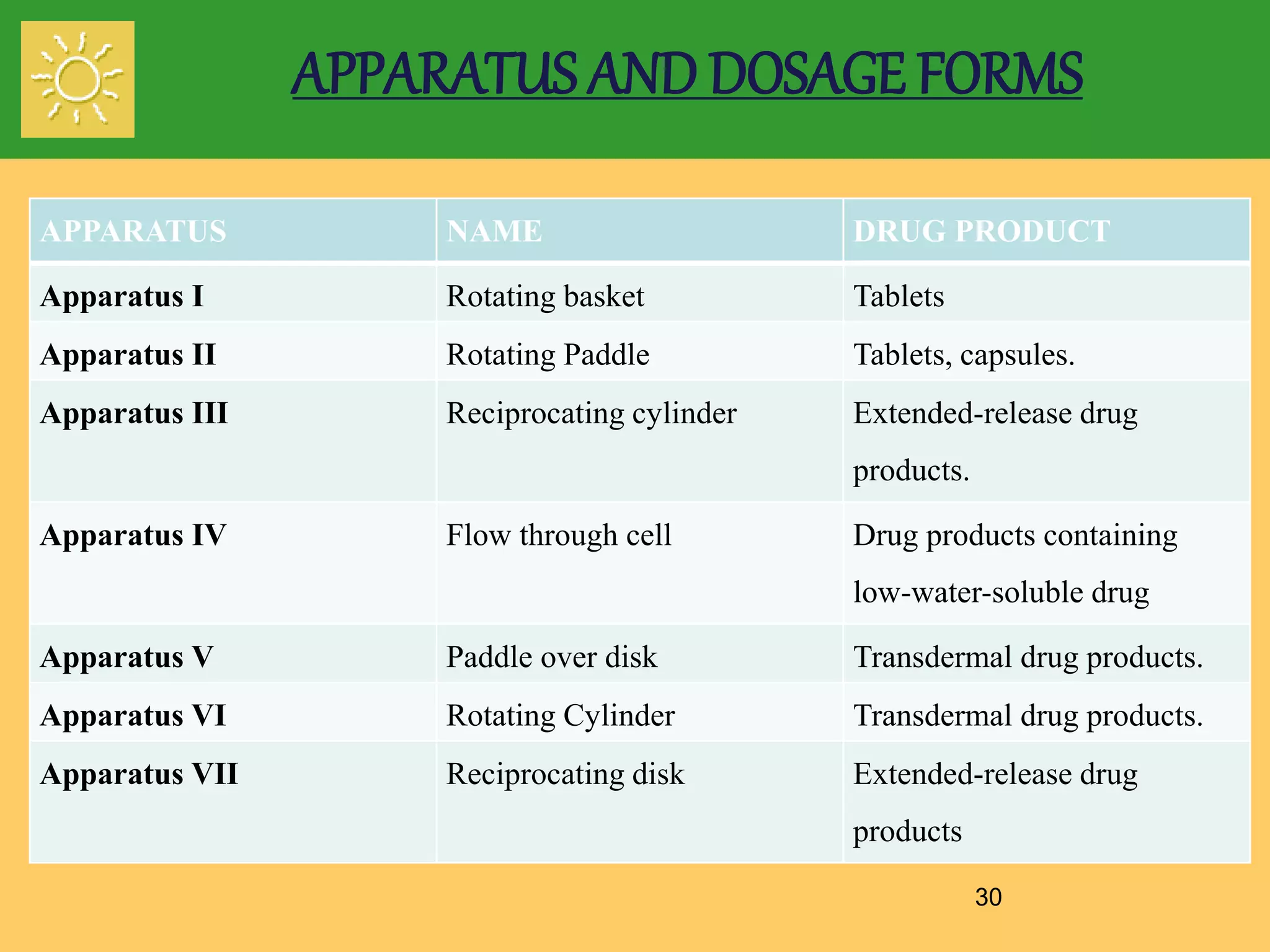
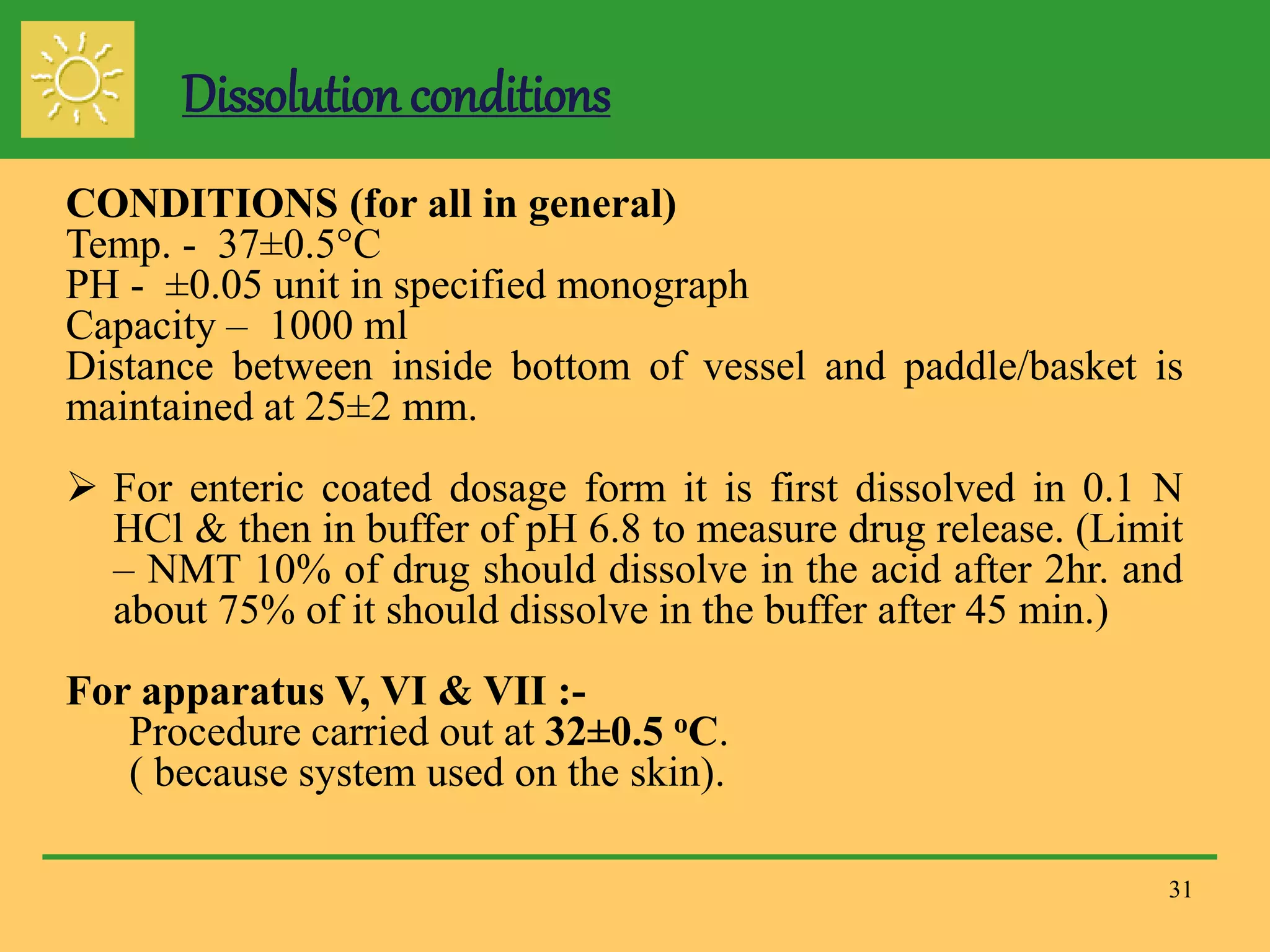
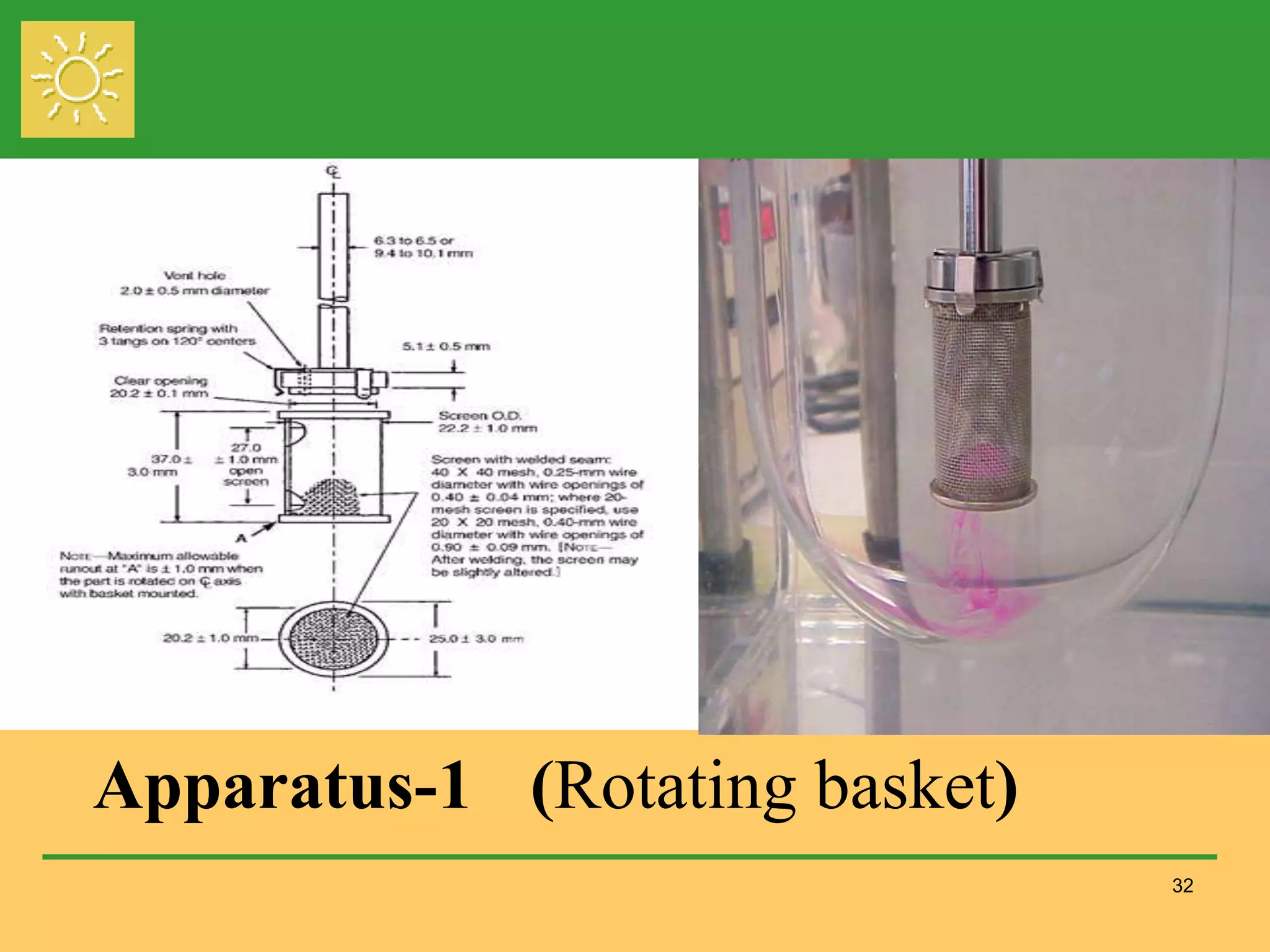
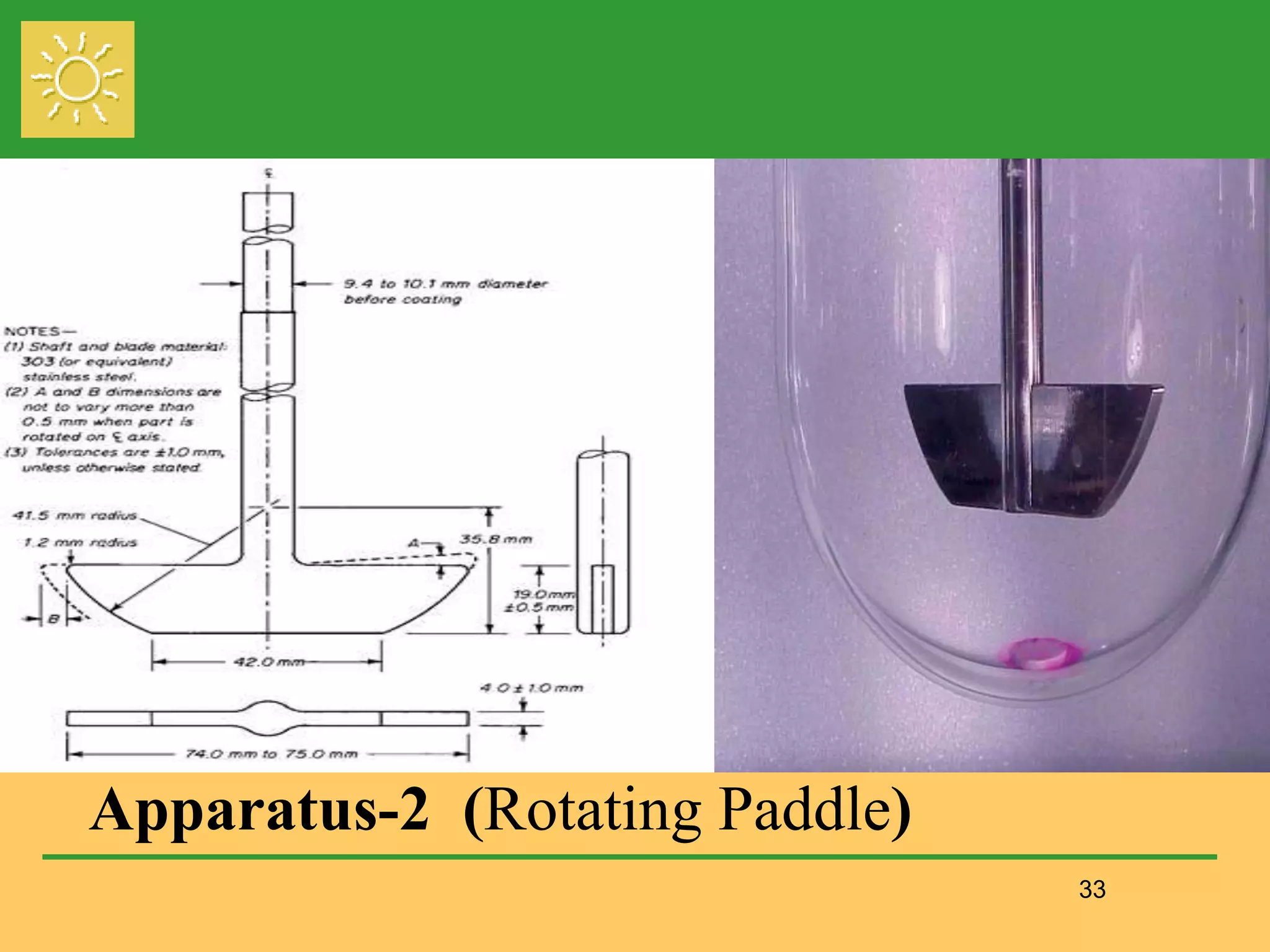
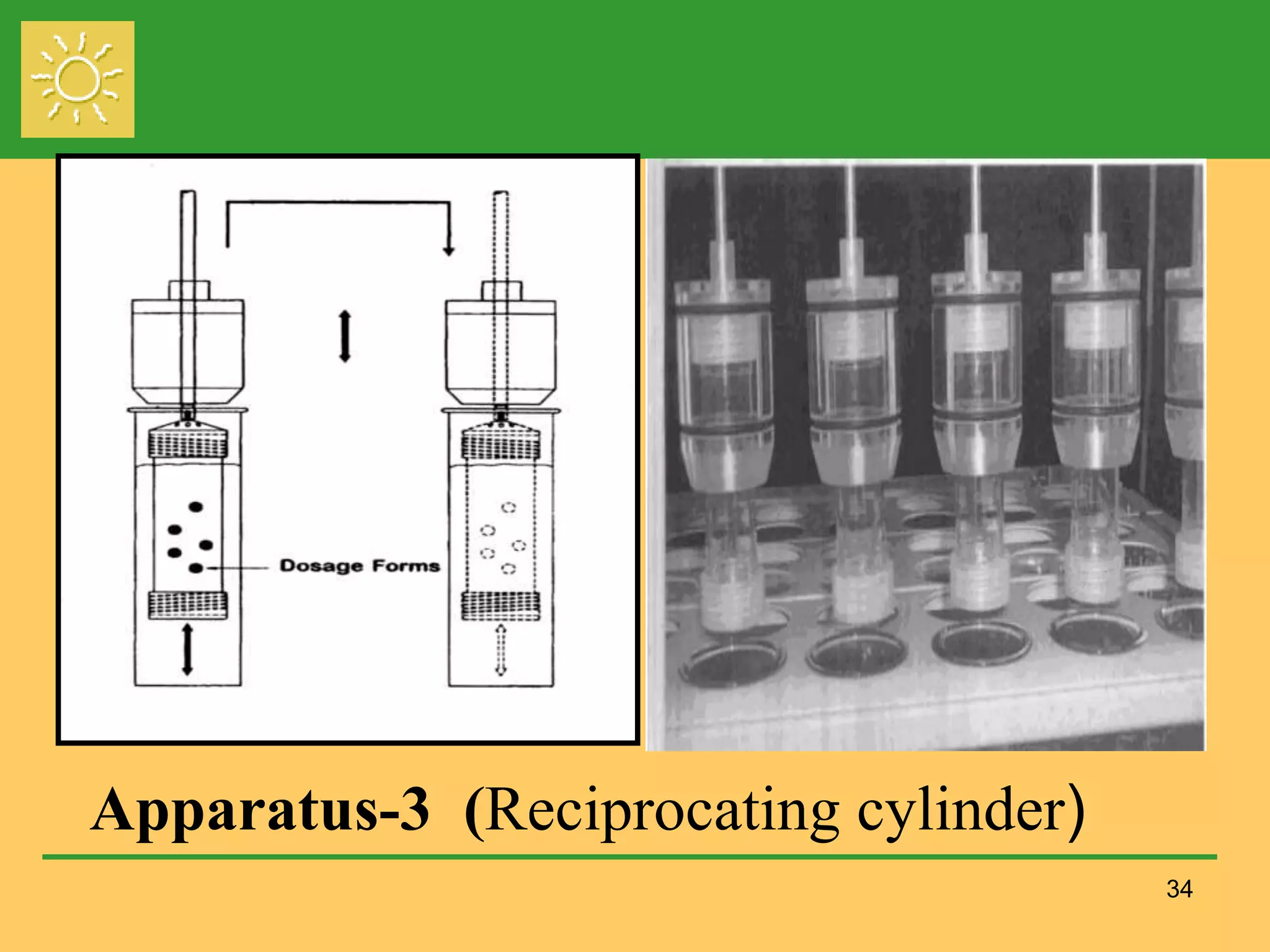
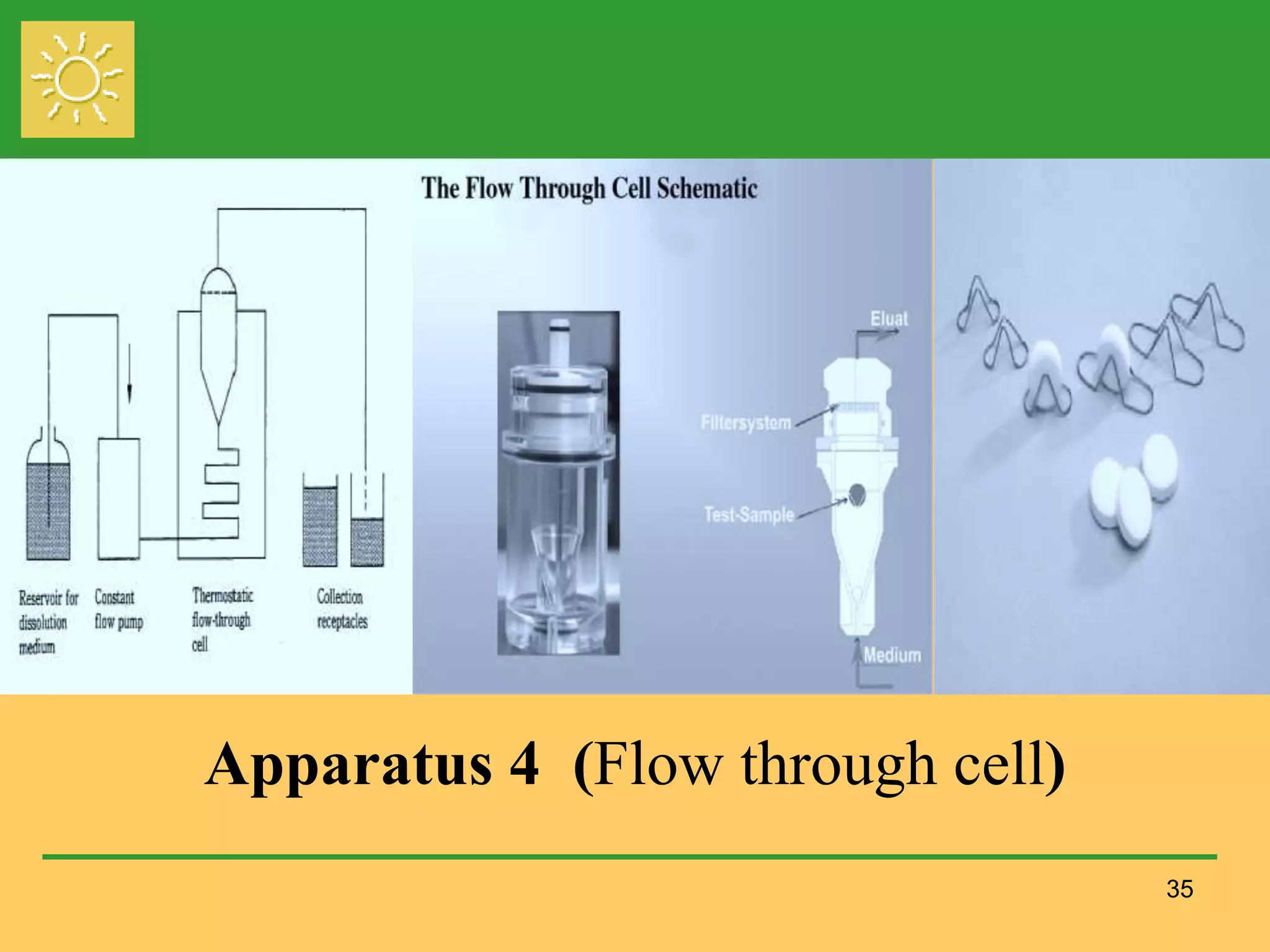
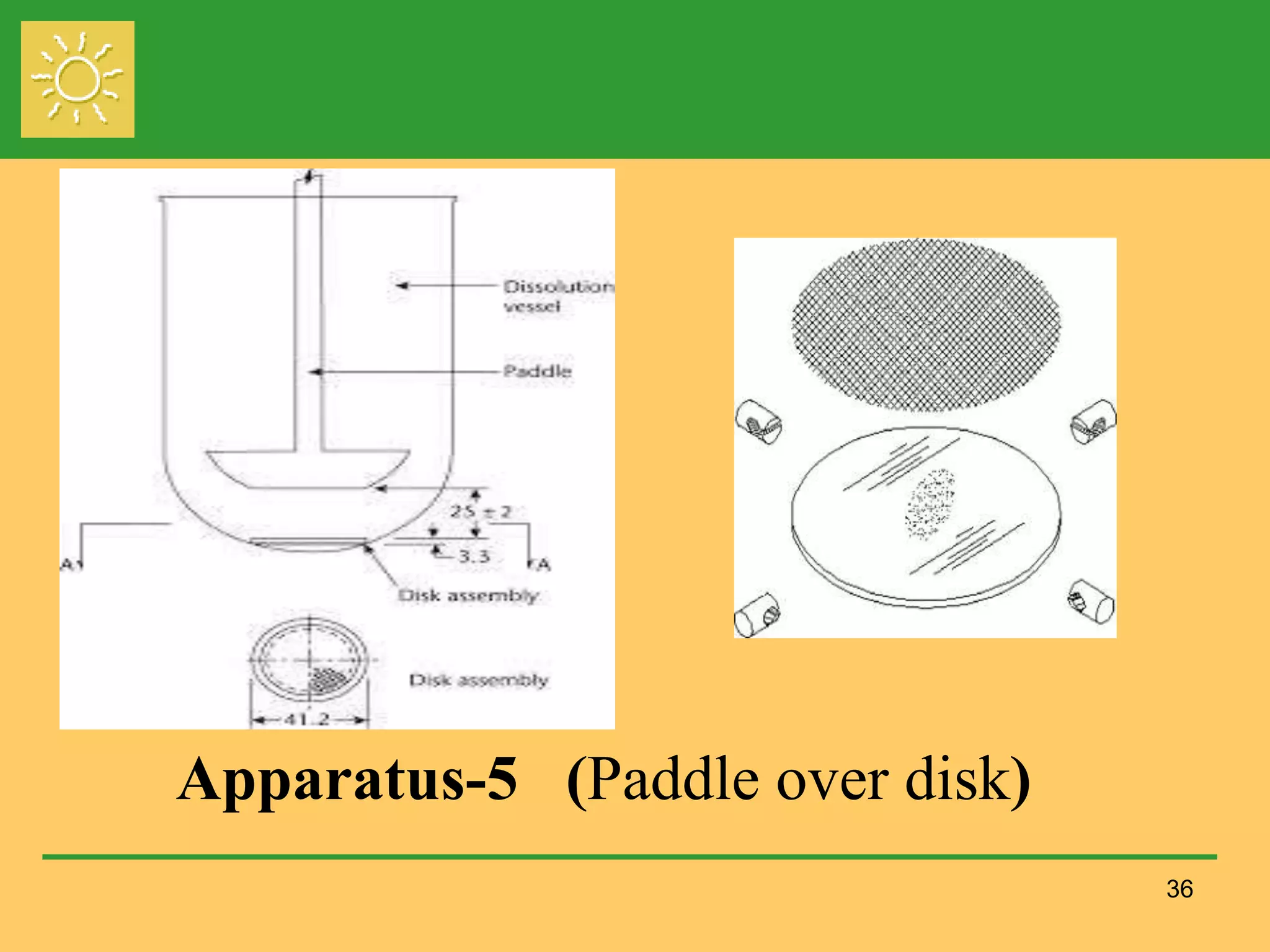
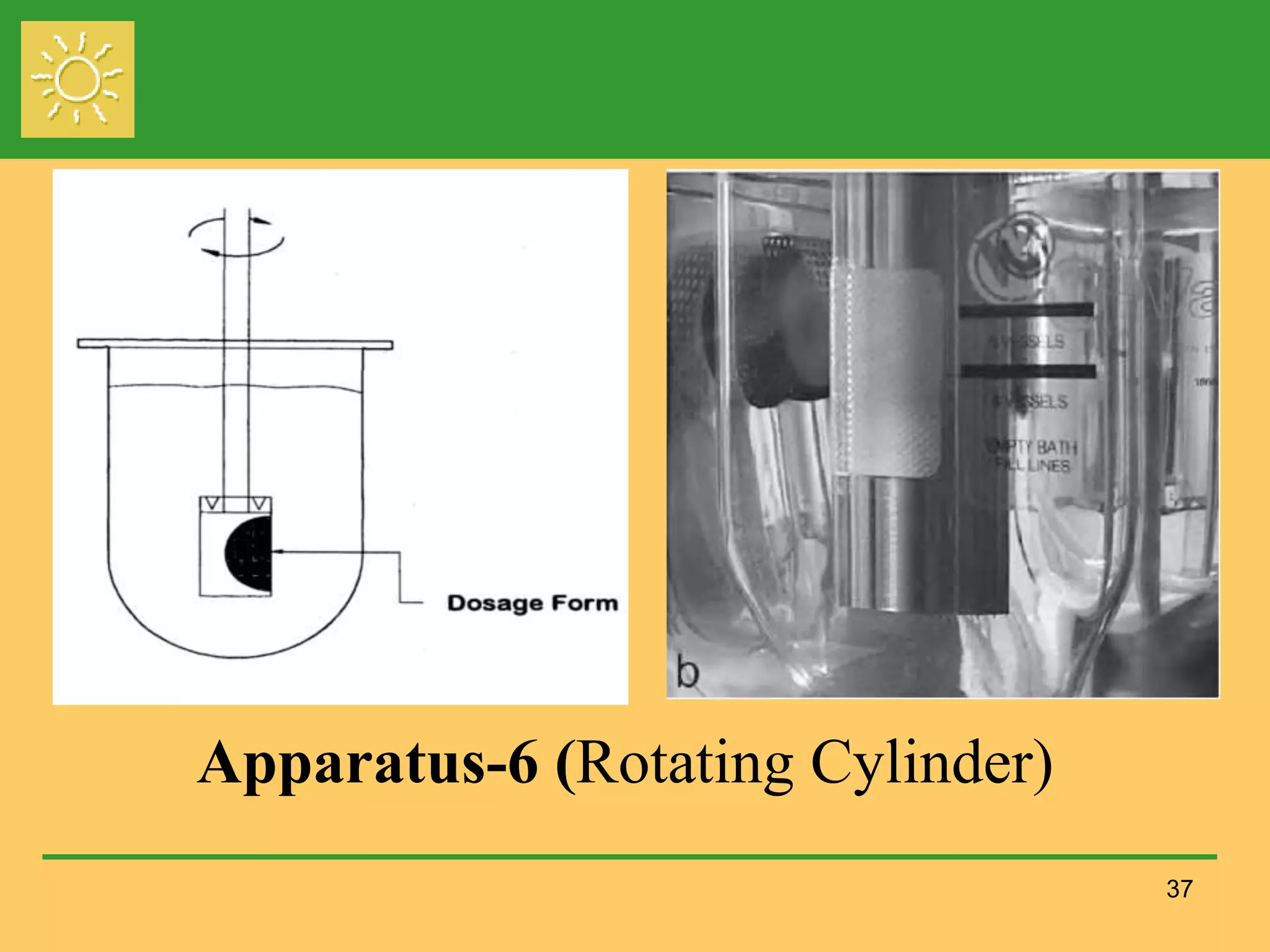
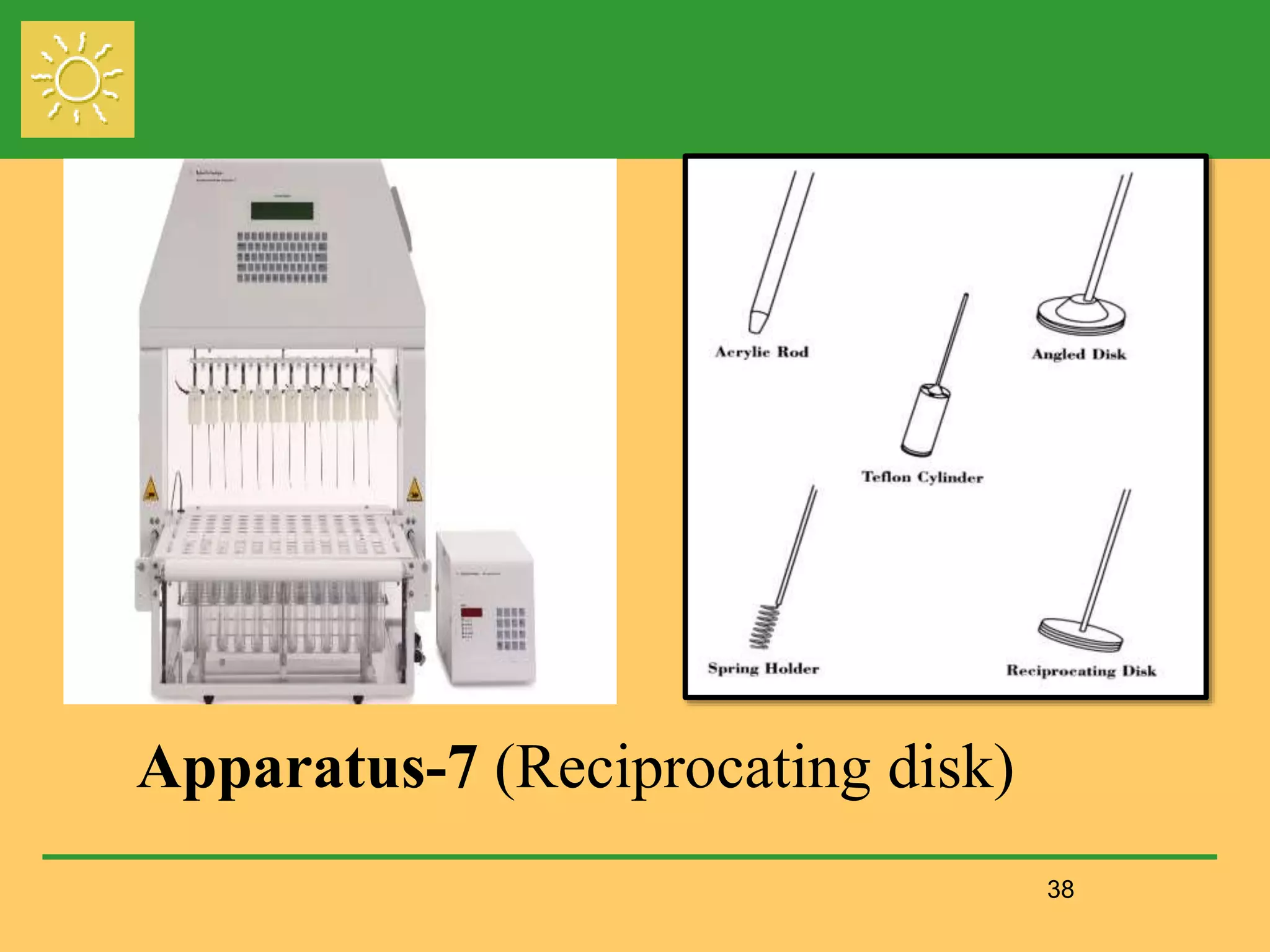
The document outlines the definition, importance, and application of drug dissolution processes, emphasizing the significance of dissolution testing in predicting bioavailability and ensuring product quality. It discusses various factors affecting dissolution rates, including physicochemical properties of drugs, formulation factors, and processing conditions, alongside different dissolution theories and apparatus. Additionally, it highlights the importance of maintaining standardized conditions in dissolution testing to ensure reliable results.