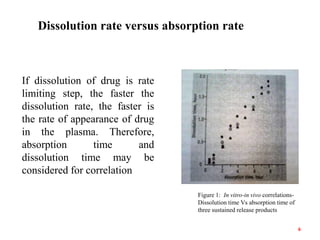
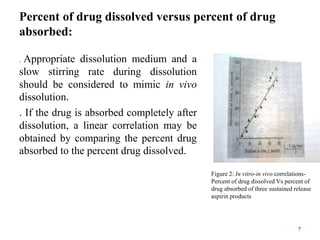
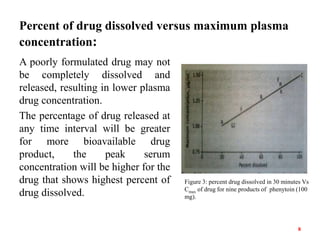
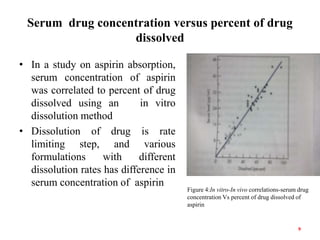
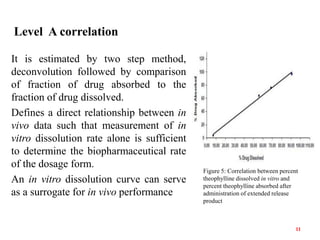
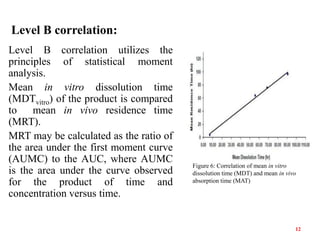
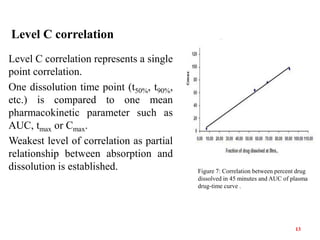
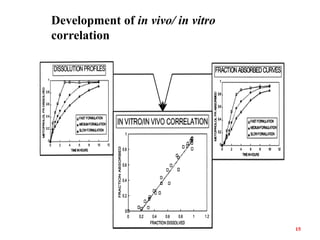
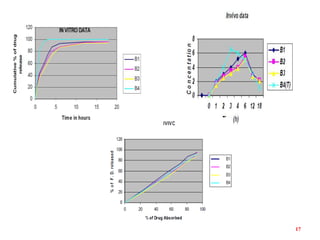
This document discusses in vitro-in vivo correlations (IVIVCs). It defines IVIVC as a predictive mathematical model relating an in vitro property (e.g. dissolution rate) to an in vivo response (e.g. absorption rate). The document outlines the significance of IVIVCs in reducing bioequivalence studies and supporting biowaivers. It describes different levels of IVIVC (A, B, C) and parameters that can be correlated (dissolution rate to absorption rate; percent dissolved to percent absorbed). The document provides examples of IVIVC case studies and concludes that current regulatory guidelines only apply to oral dosage forms, while further research is needed to develop IVIVCs for other drug products.