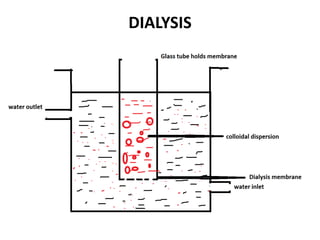
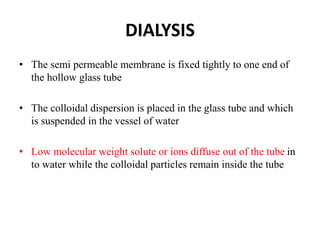
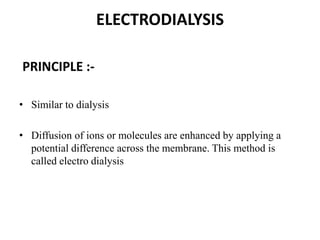
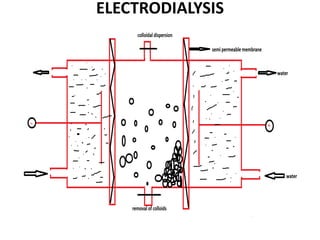
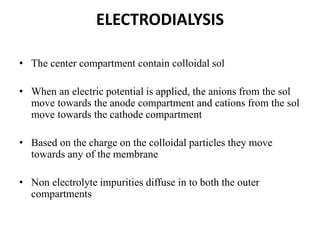
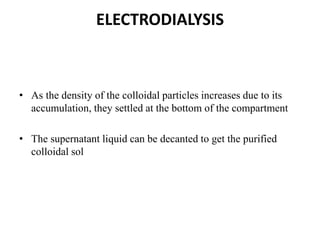
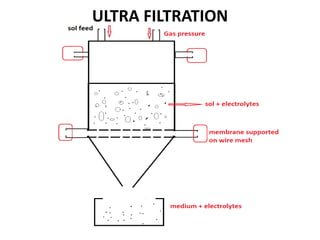
This document discusses different methods for purifying colloidal dispersions, including dialysis, electrodialysis, and ultrafiltration. Dialysis involves diffusing low molecular weight impurities out of a colloidal solution through a semi-permeable membrane. Electrodialysis enhances this diffusion process by applying an electric potential. Ultrafiltration uses an ultrafilter membrane with small pores to retain colloidal particles while filtering out smaller solutes under pressure.