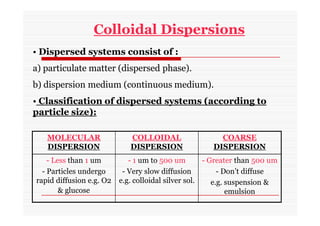
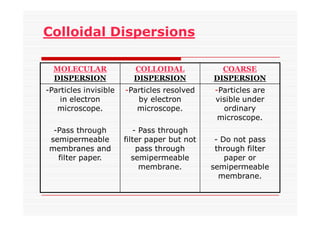
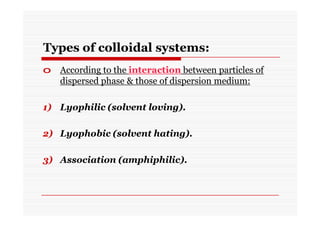
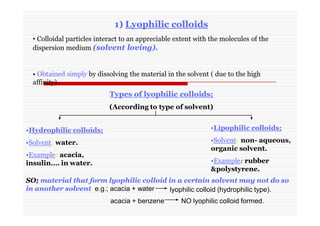
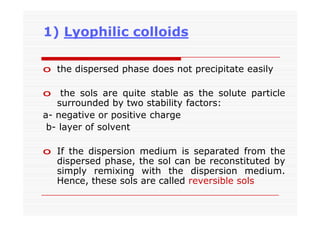
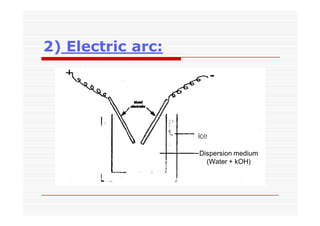
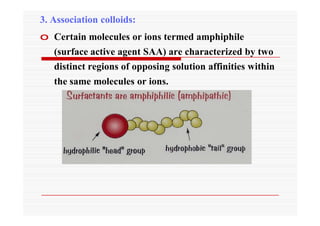
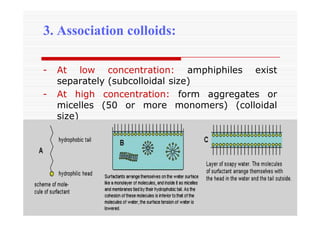
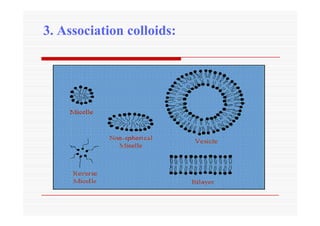
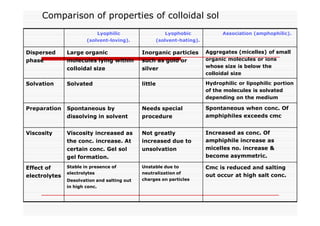
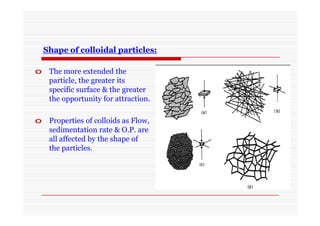
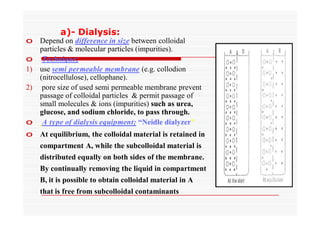
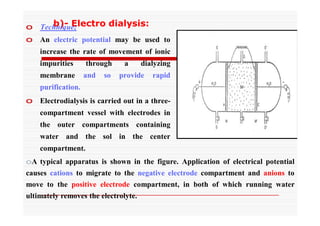
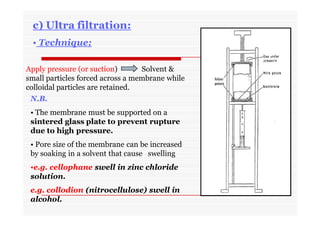
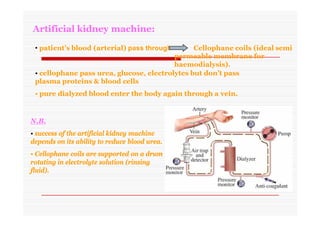
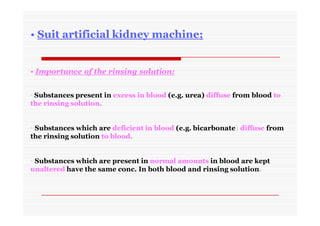
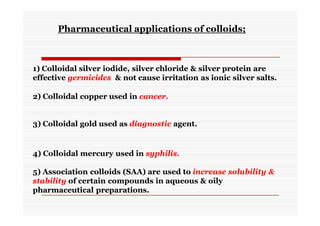
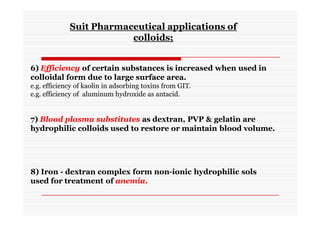
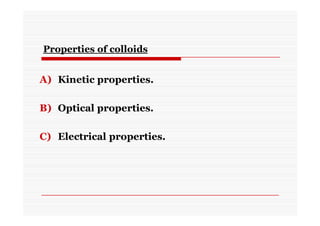
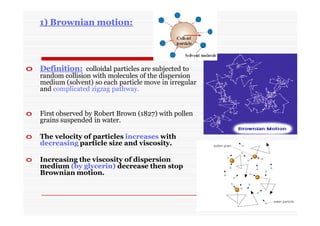
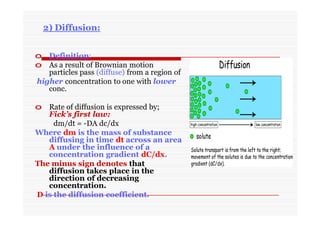
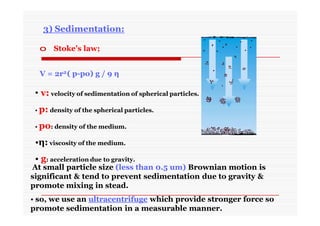
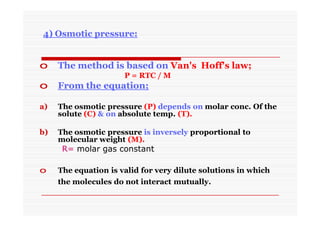
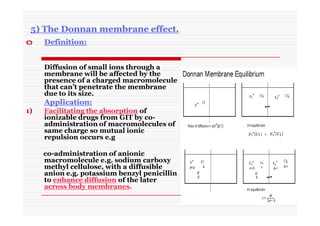
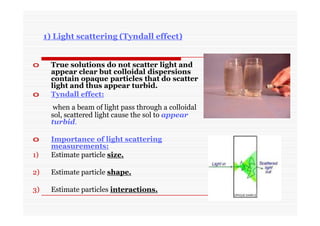
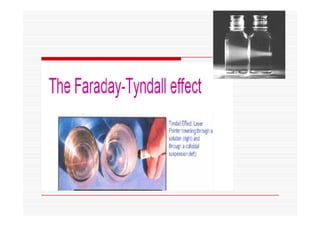
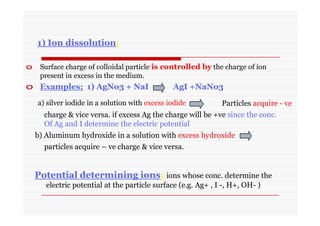
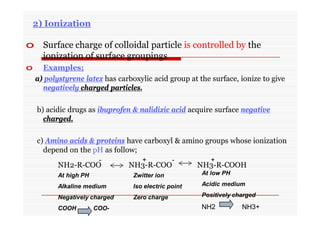
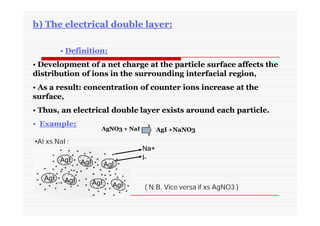
The document discusses colloidal dispersions, which are systems with particulate matter dispersed in a continuous medium. Colloidal dispersions have particle sizes between 1-500 nm. They are classified as lyophilic, lyophobic, or association colloids based on particle interactions. Lyophilic colloids are stable and interact with the solvent, while lyophobic colloids are less stable and do not interact well with water. Association colloids form micelles above a critical concentration of amphiphilic molecules. Colloids have various properties related to their kinetics, optics, and electrical characteristics that depend on factors like particle size, shape, and surface charge.