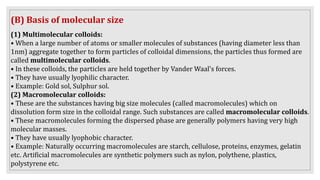
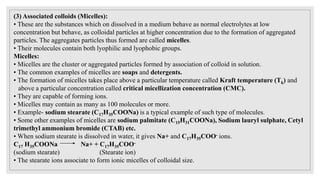
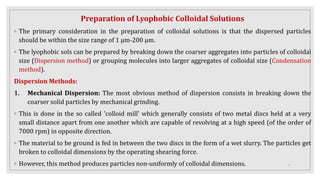
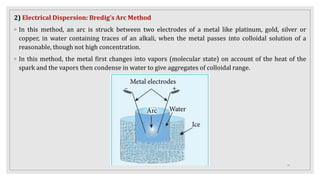
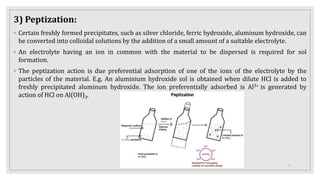
This document provides information about colloids, including:
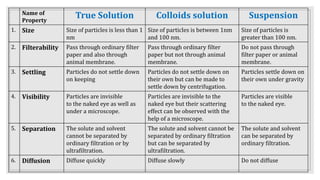
1. It defines colloids and distinguishes them from true solutions and suspensions based on particle size and other properties.
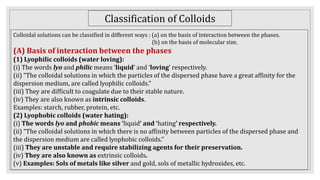
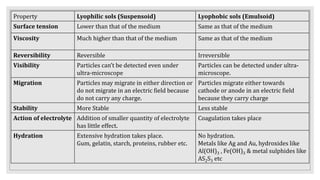
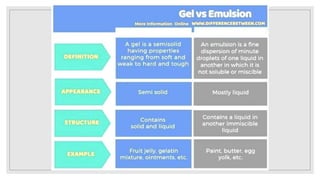
2. It describes the phases in a colloidal solution and different classification methods for colloids.
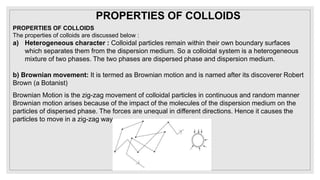
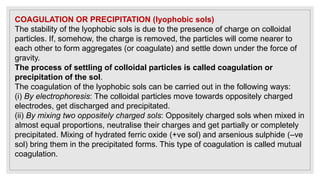
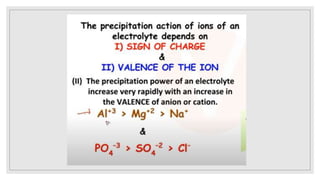
3. It explains various preparation methods for lyophobic colloidal solutions and properties of colloids such as Brownian motion, Tyndall effect, and coagulation.
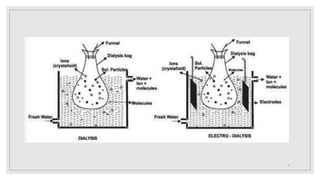
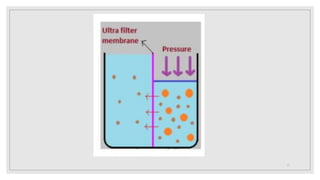
4. It discusses purification techniques like dialysis and ultrafiltration and industrial applications of colloids.