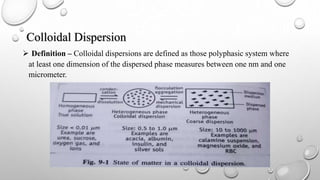
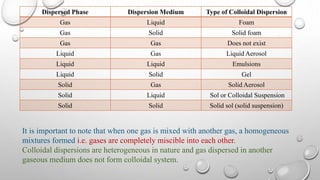
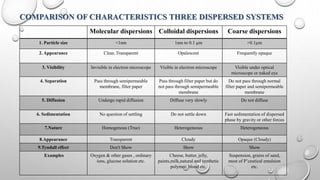
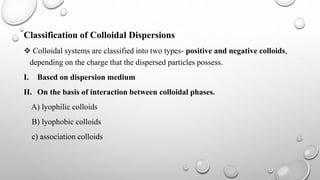
Colloidal dispersions are heterogeneous systems where one substance is divided into small particles, between 1 nm and 1 μm, dispersed throughout a second substance. They can be classified based on the dispersed and dispersion medium, such as sols where the medium is a liquid. Colloidal particles exhibit properties like Brownian motion, diffusion, and Tyndall effect. Their size, shape, and surface charge affect characteristics like stability, flowability, and pharmacological effects. Purification methods remove electrolytes and impurities through dialysis, ultrafiltration, or electrodialysis.