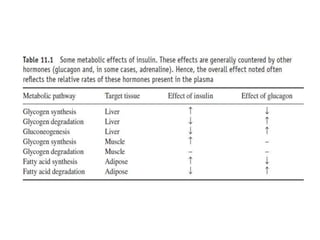
This document discusses various therapeutic hormones including insulin, growth hormone, gonadotrophins, thyroid stimulating hormone, parathyroid hormone, and calcitonin. It provides details on their structure, function, production, formulations, and medical applications. Key points include: insulin is produced in the pancreas and regulates blood glucose; growth hormone stimulates growth; gonadotrophins like FSH and LH regulate reproduction; recombinant DNA technology is now used to produce many therapeutic hormones which has improved safety over extracts from animal tissues. These hormones are administered to treat various endocrine disorders and fertility issues.