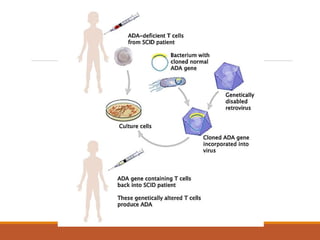
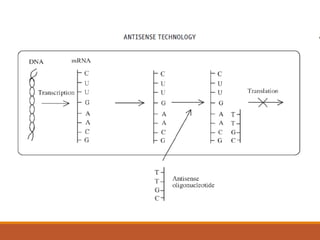
Nucleic acid and cell based therapies involve gene therapy and cell therapy. Gene therapy aims to introduce new genes into cells to treat genetic diseases by replacing defective genes. Early gene therapy trials focused on ex vivo and in vivo approaches. Viral vectors like retroviruses were primarily used but posed safety risks. Non-viral methods using naked DNA or vectors like liposomes were developed. Diseases targeted include cancer, genetic disorders, and AIDS. Antisense oligonucleotides can bind mRNA and inhibit gene expression. RNA interference uses short interfering RNAs to induce sequence-specific gene silencing. Ribozymes and aptamers also provide nucleic acid based therapeutic approaches.