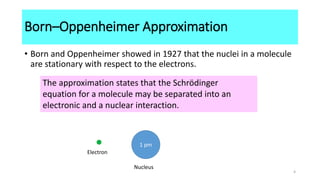
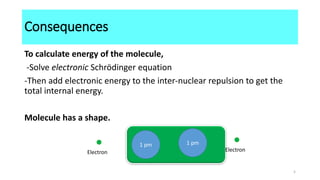
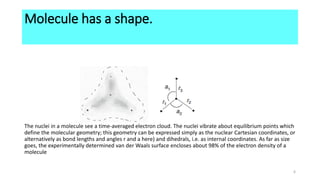
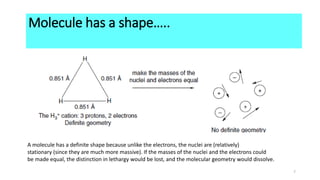
The Born-Oppenheimer approximation, proposed in 1927 by physicists Max Born and J. Robert Oppenheimer, treats the motions of nuclei and electrons in molecules separately. It approximates that the nuclei in a molecule are stationary relative to the rapidly moving electrons. This allows molecular structure and properties to be determined by first solving the electronic Schrodinger equation at fixed nuclear positions, and then adding the internuclear repulsion energy to obtain the total internal energy of the molecule. As a result of this approximation, molecules have well-defined shapes determined by the equilibrium positions of their nuclei.