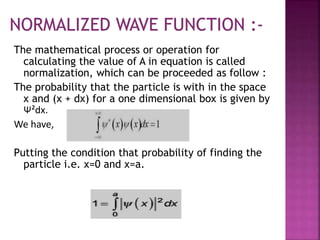
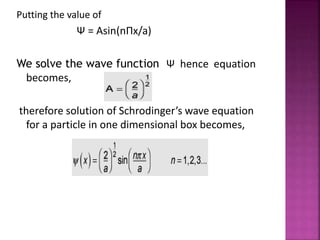
This document discusses the Schrodinger wave equation and its application to modeling a particle in a one-dimensional box. It introduces the history and derivation of the Schrodinger equation, then applies it to solve for the energy and wave functions of a particle confined to a box. The energy is found to be quantized and depend on an integer quantum number. Graphs of the wave functions and probability densities are presented.



![ E is a energy wave function and their value
are:-
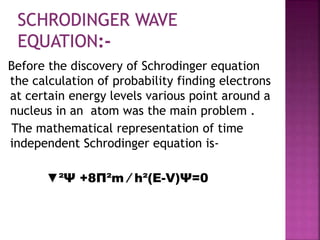
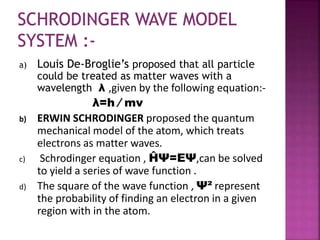
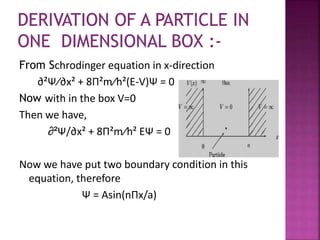
[ E =n²h²/8ma² ]
This equation shows it is energy of the particle
in one- dimensional box in other words
energy depends upon the quantum number
‘n’ which have any integral value the energy
level of its particle in a box are quantized.](https://image.slidesharecdn.com/one-dimensionalbox-200229171544/85/One-dimensional-box-8-320.jpg)