This document discusses the statistical mechanics of molecular systems. It introduces key concepts like molecular configurations, Boltzmann distribution, and molecular partition functions. The Boltzmann distribution relates the probability of finding a molecule in a particular energy state to the state's energy and temperature. It can be derived using the method of Lagrange multipliers to maximize the multiplicity of configurations under energy and particle number constraints. The molecular partition function indicates the number of accessible energy states and approaches the ground state degeneracy as temperature approaches zero and the total number of states as temperature increases infinitely.





















![GHC@copyright
When N is even, the weight is maximum at k = N/2,
i.e.
Wk=N/2 = N! / [N/2)!]2.
When N is odd, the maximum is at k = N/2 1
As N increases, the maximum becomes sharper!
The weight for k = N/4 is
Wk=N/4 = N! / [(N/4)! (3N/4)!]
The Dominating Configuration](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-23-320.jpg)
![GHC@copyright
| N | 4 | 8 | 16 | 32 | 256 | 6.0 x 1023
|R(N) | 1.5 | 2.5 | 7.1 | 57.1 | 3.5 x 1014 | 2.6 x 103e+22
Therefore, for a macroscopic molecular system
( N ~ 1023 ), there are dominating configurations
so that the system is almost always found in or
near the dominating configurations, i.e. Equilibrium
The ratio of the two weights is equal to
R(N) Wk=N/2 / Wk=N/4
=(N/4)! (3N/4)! / [(N/2)!]2
The Dominating Configuration
If the system has more states, would we reach the same
conclusion as above? Why?](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-24-320.jpg)

![GHC@copyright
The Boltzmann Distribution
ni / N = Pi = exp ( - i )
Interpretation of Boltzmann Distribution
Meaning of : ensure total probability is ONE
1 = i ni / N = i exp( -i)
exp( ) = 1 / i exp(-i)
= - ln [i exp(-i)]
1 / = kT](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-26-320.jpg)





















![GHC@copyright
JUSTIFICATION
Statistical Thermodynamics
The energy of a molecular system U can be expressed as,
U = U(0) + i nii
where, U(0) is the internal energy of the system at T=0,
ni is the number of molecules which are in the state with
its energy equal to i
Now let’s imagine that the system is being heated while
the volume V is kept the same. Then the change of U
may be written as,
dU = dU(0) + i nidi + i idni = i idni
[dU(0) = 0 because U(0) is a constant; di = 0 because i
does not change as the temperature of the system arises.]](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-48-320.jpg)




![GHC@copyright
The relation between S and the partition function q
Statistical Thermodynamics
According to the Boltzmann distribution,
ln pi = - i - ln q
Therefore,
S = - Nk i pi (- i - ln q)
= k i ni i + Nk ln qi pi
= E / T + Nk ln q
= [U-U(0)] / T + Nk ln q
This relation may be used to calculate S from the known entropy q](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-53-320.jpg)

![GHC@copyright
The partition function Q
Q = i exp[-i(1) - i(2) - i(3) - … -i(N)]
= {i exp[-i(1)]}{i exp[-i(2)]} … {i exp[-i(N)]}
= {i exp(-i)}N
= qN
where q i exp(-i) is the molecular partition function. The
second equality is satisfied because the molecules are independent
of each other.
Statistical Thermodynamics](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-55-320.jpg)
![GHC@copyright
The relation between U and the partition function Q
U = U(0) - (lnQ/)V
The relation between S and the partition function Q
S = [U-U(0)] / T + k ln Q
The above two equations are general because they not only apply to
independent molecules but also general interacting systems.
Statistical Thermodynamics](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-56-320.jpg)


![GHC@copyright
Setting the lowest energy to zero, the relative energies
can then be expressed as,
qx = 1 dn exp [ -(n2-1) ] = 1 dn exp [ -(n2-1) ]
= 0 dn exp [ -n2 ] = (2m/h22)1/2 X
n = (n2-1) with = h2 / (8mX2)
qx = n exp [ -(n2-1) ]
is very small, then
Statistical Thermodynamics](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-59-320.jpg)


![GHC@copyright
Fundamental Thermodynamic Relationships
Consider an equilibrium system which is consistent of N
interacting molecules. These molecules may or may not
be the same.
Relation between energy and partition function
Statistical Thermodynamics
U = U(0) + E = U(0) - (lnQ/)V
Relation between the entropy S and the partition function Q
S = [U-U(0)] / T + k lnQ](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-62-320.jpg)











![GHC@copyright
Statistical Thermodynamics
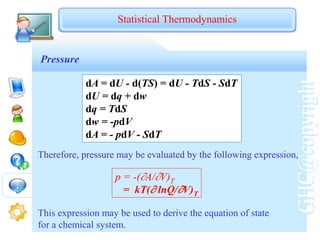
Factorization of Molecular Partition Function
i
E
i
V
i
R
i
T
i
i i
q )]
(
exp[
)
exp(
i i i i
E
i
V
i
R
i
T
i )]
exp(
)][
exp(
)][
exp(
)][
exp(
[
E
V
R
T
q
q
q
q
](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-75-320.jpg)

![GHC@copyright
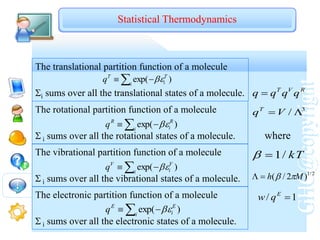
Then the molecular partition function can be evaluated
n
n n
v
hv
hv
n
q )]
exp(
1
/[
1
)
exp(
)
exp(
...
1 3
2
e
e
e
qv
1
....
3
2
v
v
q
e
e
e
q
e
hv
v
e
e
q
1
1
)
1
/(
1
Consider the high temperature situation where kT >>hv, i.e.,
hv
kT
hv
hv v
/
/
1
q
,
1
Vibrational temperature v
High temperature means that T>>v
hv
k v
h
e hv
1
I2 F2 HCl H2
v/K 309 1280 4300 6330
v/cm-1 215 892 2990 4400
m
k
v
where
Therefore,
Vibrational Partition Function](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-78-320.jpg)
![GHC@copyright
where B is the rotational constant. J =0, 1, 2, 3,…
)
1
(
J
hcBJ
R
J
states
rotational
all
]
exp[ R
J
R
q
levels
energy
rotational
all
]
exp[ R
J
J
g
J
)]
1
(
exp[
)
1
2
( J
hcBJ
J
0
)]
1
(
exp[
)
1
2
( dJ
J
hcBJ
J
qR
dJ
/
)]}
1
(
{exp[
)
/
1
(
0
dJ
J
hcBJ
d
hcB
0
]}
1
(
){exp[
/
1
( l
J
hcBJ
hcB
Bh/8cI2
c: speed of light
I: moment of Inertia
i
i
r m I
i
2
hcB<<1
where gJ is the degeneracy of rotational energy level εJ
R
Usually hcB is much less than kT,
Note: kT>>hcB
Rotational Partition Function
If we may treat a heteronulcear diatomic molecule as a rigid rod,
besides its vibration the two atoms rotates. The rotational energy
=kT/hcB](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-79-320.jpg)

![GHC@copyright
where, gE = g0 is the degeneracy of the electronic ground state,
and the ground state energy 0
E is set to zero.
If there is only one electronic ground state qE = 1, the partition
function of a diatomic gas,
]
exp[
]
exp[
states
electronic
all energies
electronic
all
E
j
j
E
j
E
g
q
]
exp[ 0
0
E
g
N
hv
N
N
e
hcB
kT
V
N
Q
)
1
(
)
/
(
)
/
)(
!
/
1
( 3
At room temperature, the molecule is always in its ground state
Electronic Partition Function
=g0
=gE](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-81-320.jpg)
![GHC@copyright
The internal energy of a diatomic gas (with N molecules)
v
e
n
N
N
n
N
U
U hv
v
v ]
/
)
1
(
1
[
)
/
ln
(
)
/
1
(
3
)
0
(
)
1
/(
/
1
/
1
)
2
/
3
(
hv
e
Nhv
N
N
)
1
/(
)
2
/
5
(
hv
e
Nhv
NkT
kT
N
)
2
/
7
(
qV = kT/hv
qR = kT/hcB
The rule: at high temperature, the contribution of one
degree of freedom to the kinetic energy of a molecule
(1/2)kT
Mean Energy and Heat Capacity
(T>>1)](https://image.slidesharecdn.com/chem2503oct05-230505152643-b7988c69/85/chem2503_oct05-ppt-82-320.jpg)







