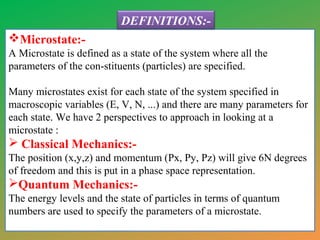
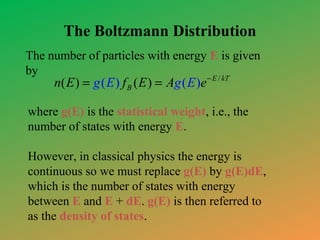
This document provides an overview of statistical mechanics. It defines microstates and macrostates, and explains that statistical mechanics studies systems with many microstates corresponding to a given macrostate. The Boltzmann distribution is derived, which gives the probability of finding a system in a particular microstate as being proportional to the exponential of the negative of the energy of that microstate divided by the temperature. Maxwell-Boltzmann statistics are described as applying to classical distinguishable particles, yielding the Maxwell-Boltzmann distribution. References for further reading are also included.