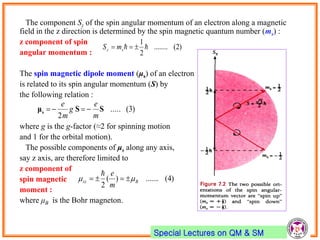
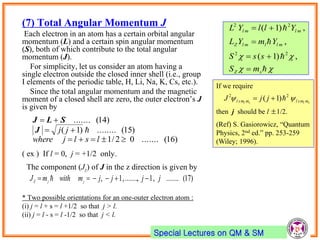
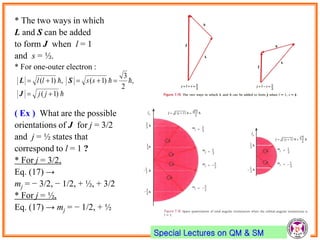
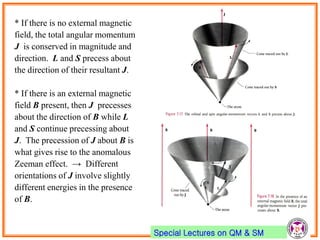
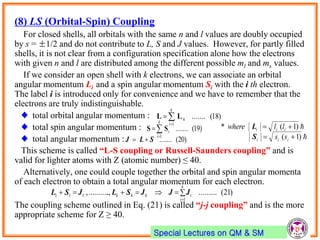
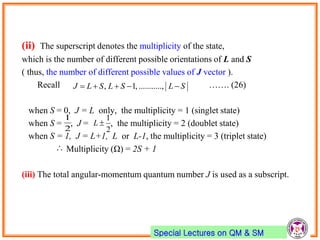
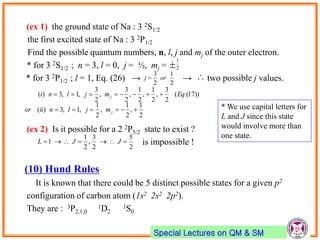
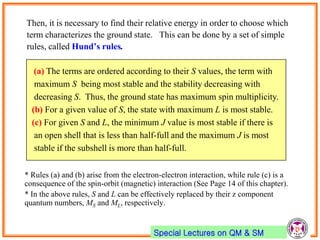
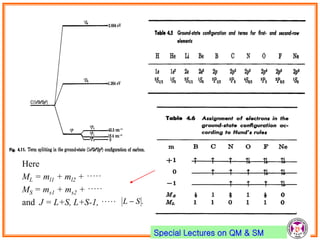
- Electrons have intrinsic angular momentum called spin. Spin takes values of ±1/2.
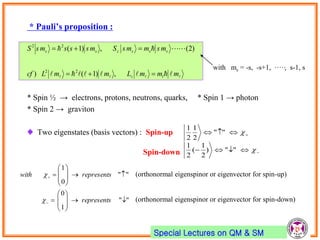
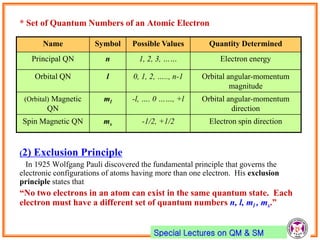
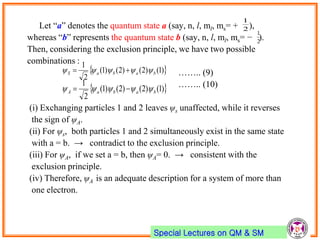
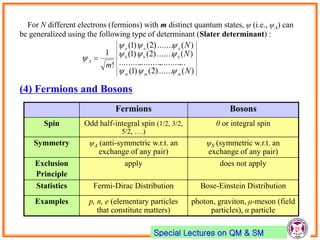
- Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers (n, l, ml, ms).
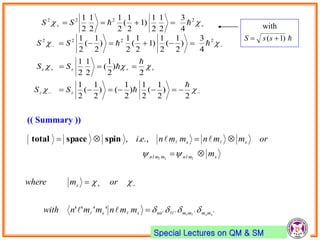
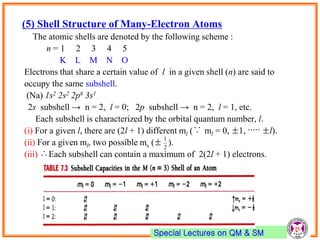
- Atomic orbitals are characterized by principal quantum number n, azimuthal quantum number l, and magnetic quantum number ml.
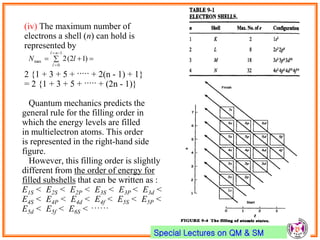
- Electrons first fill up lowest energy orbitals according to Aufbau principle.
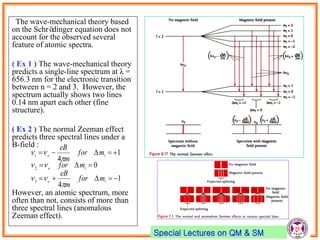
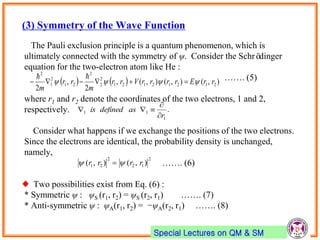
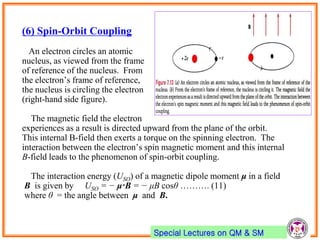
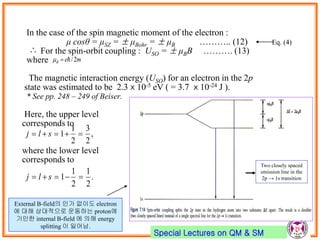
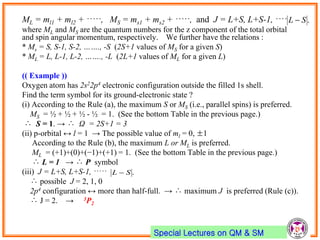
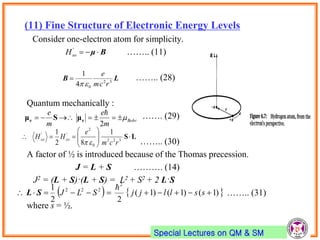
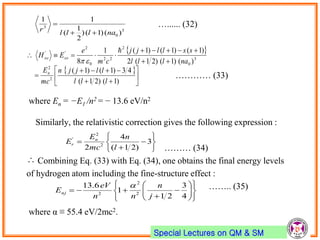
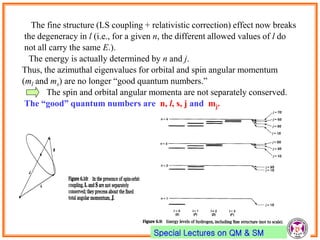
- Spin-orbit coupling arises from the interaction of an electron's magnetic moment with the magnetic field generated by the nucleus. This leads to splitting of energy levels.