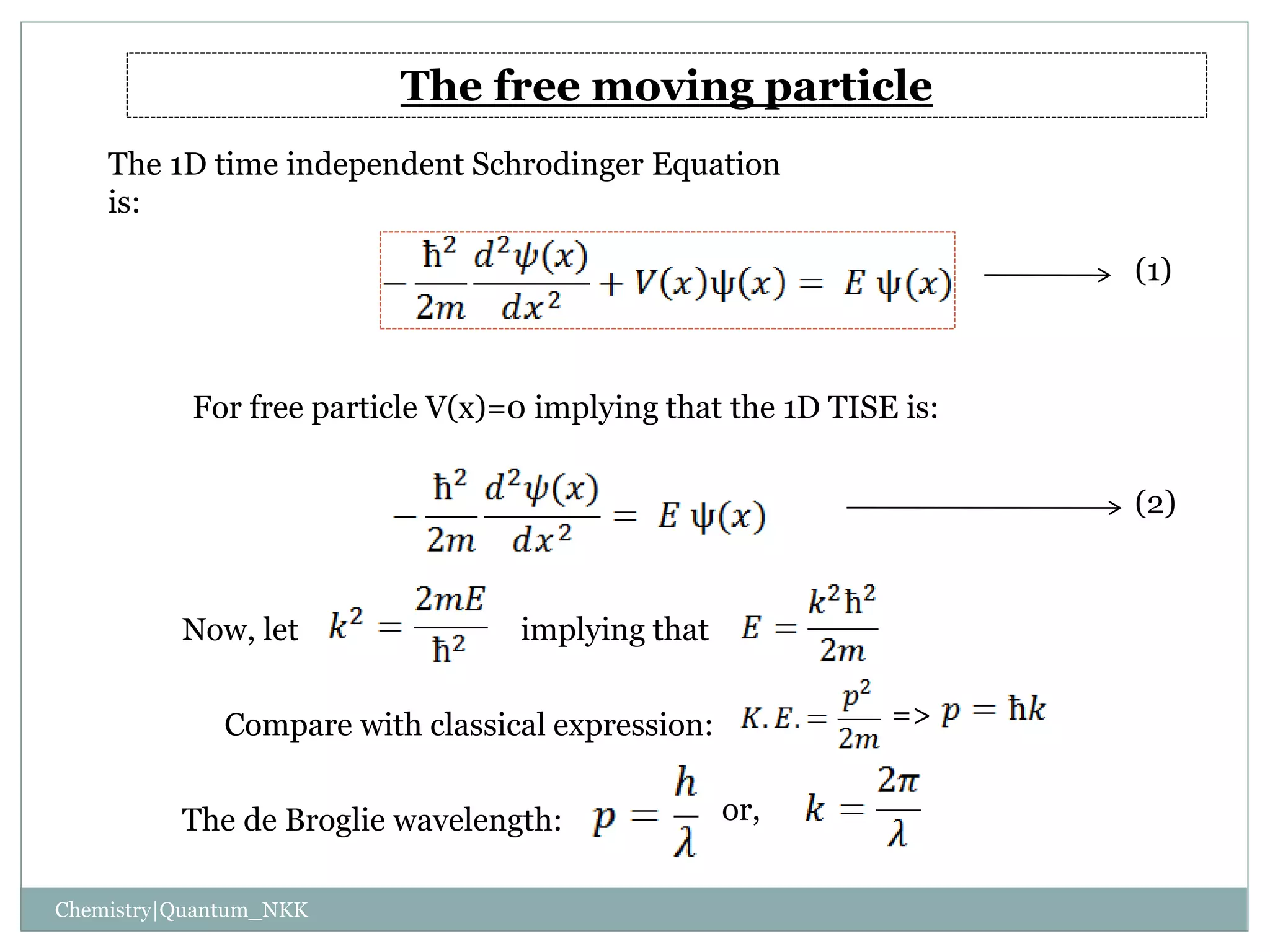
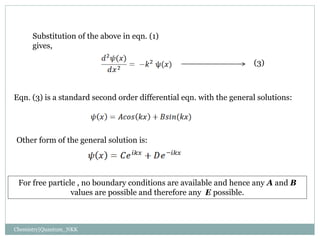
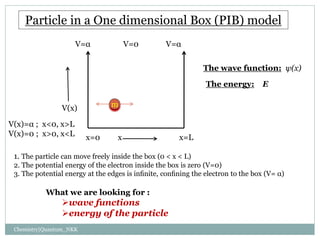
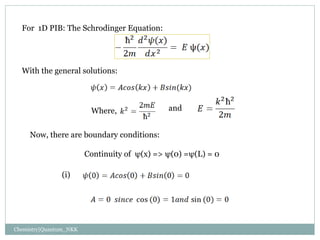
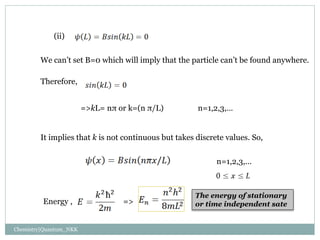
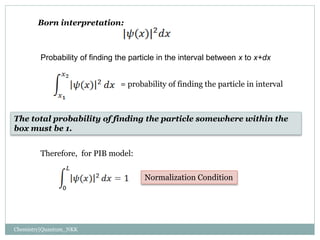
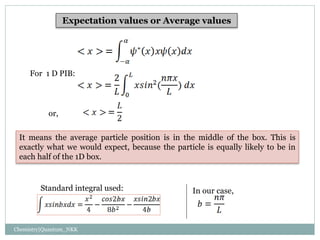
1. The particle is confined to a one-dimensional box of length L, with potential energy V=0 inside the box and V=infinity outside.
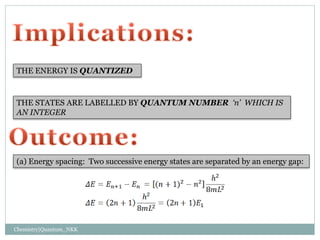
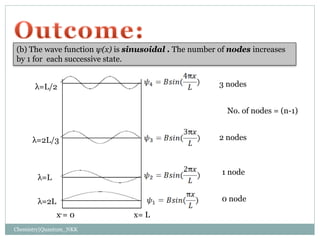
2. The wave functions and energy levels of the particle are quantized. The wave functions are sinusoidal with n nodes, and the energy is proportional to n^2.
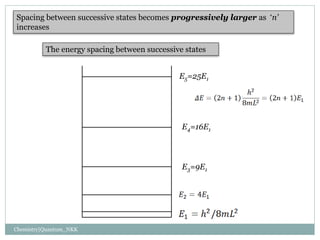
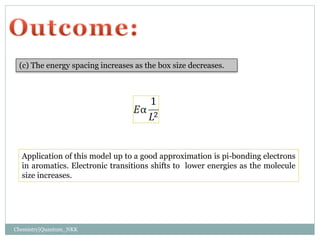
3. The energy levels are spaced further apart at higher n values, with the spacing between levels increasing as the box size decreases.