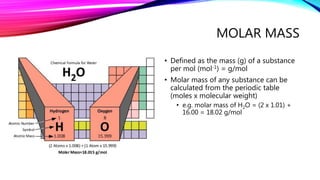
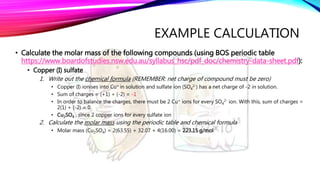
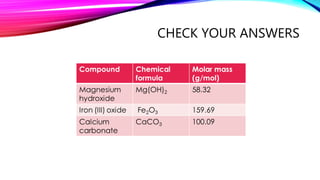
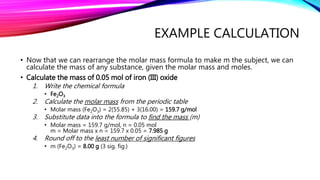
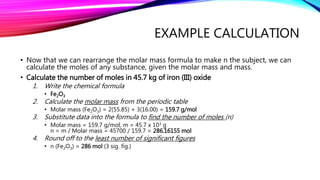
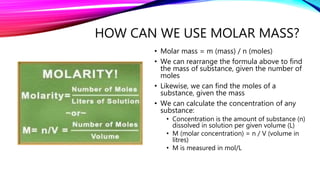
The mole is a unit for measuring the amount of substance, with 1 mole equaling 6.022 x 10^23 particles. Molar mass is calculated as the mass of a substance per mole and can be derived from the periodic table, while concentration indicates the amount of substance per volume. Understanding these concepts allows for the calculation of mass, moles, and concentration from given values.