This document provides an overview of stoichiometry concepts including:
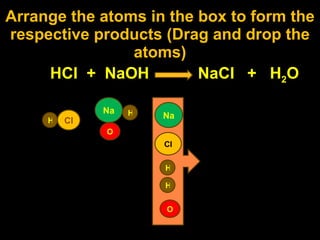
1) Balancing chemical equations and understanding coefficients and subscripts.
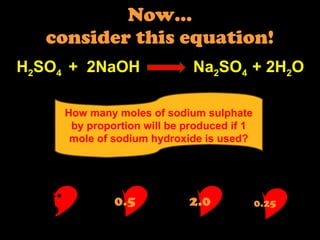
2) Determining limiting reagents and calculating product amounts based on reactant ratios.
3) Applying stoichiometric concepts to solve quantitative problems involving moles, masses, and volumes of reactants and products.










































![H 2 SO 4 + Mg MgSO 4 + H 2 Tip : You may need paper, pen and a calculator If 24g of magnesium is used in the above reaction, how many moles of hydrogen gas will be produced?[Relative atomic mass of magnesium: 24] 2 moles 0.5 mole 1 mole 3 moles](https://image.slidesharecdn.com/stoichiometry-111012004856-phpapp02/85/Stoichiometry-45-320.jpg)



![2AI + 6HCI 2AICI 3 + 3H 2 30cm 3 of hydrochloric acid with the concentration of 0.2 moldm -3 was used in this experiment. What is the volume of hydrogen gas that will be produced? [Molar volume of gas: 24 dm 3 mol -1 ] 60cm 3 0.06cm 3 72dm 3 72cm 3](https://image.slidesharecdn.com/stoichiometry-111012004856-phpapp02/85/Stoichiometry-49-320.jpg)
![C 2 H 4 + 3O 2 2CO 2 + 2H 2 O What is the minimum volume of oxygen gas is required for you to produce 0.6 moles of carbon dioxide gas? [molar volume of gas: 24 dm 3 mol -1 ] 0.9 dm 3 22 dm 3 21.6 dm 3 0.3 dm 3](https://image.slidesharecdn.com/stoichiometry-111012004856-phpapp02/85/Stoichiometry-50-320.jpg)












































![Explain excess and limiting reagent NaCI + AgNO 3 AgCI + NaNO 3 n NaCI = Mass/ RMM = 25/(23 + 35.5) = 0.42 mol n AgNO3 = Mass/ RMM = 50/[108+14+3(16)] = 0.29 mol The limiting reagent in this experiment is Silver nitrate as the number of moles of it used is fewer than the number of moles of sodium chloride used](https://image.slidesharecdn.com/stoichiometry-111012004856-phpapp02/85/Stoichiometry-96-320.jpg)

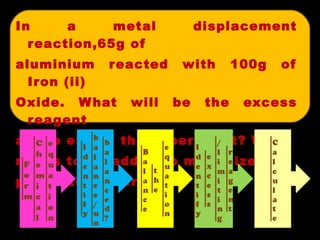



![Explain excess and limiting reagent 2Al + Fe 2 O 3 Al 2 O 3 + 2Fe n Al = Mass/ RMM = 65/27 = 2.41 mol n =Mass/ RMM = 150/[2(56) + 3(16)] = 0.94mol The Excess reagent in this experiment is Aluminium as the number of moles of it used is more than the number of moles of Iron (II) Oxide used Fe 2 O 3](https://image.slidesharecdn.com/stoichiometry-111012004856-phpapp02/85/Stoichiometry-102-320.jpg)






![From the equation, two moles of water is produced by proportion. Meaning the number of moles of water produced is = 0.007 X 2 = 0.014mol Mass of yield, H 2 O = 0.014 mol X [2(1) + 16] gmol -1 = 0.252 g Calculate the mass of the yield n= mass/RMM So, mass = n X RMM](https://image.slidesharecdn.com/stoichiometry-111012004856-phpapp02/85/Stoichiometry-109-320.jpg)



![n = MV n HCI = MV = [(1.0 mol/dm 3 ) (50 cm 3 )]÷1000 = 0.05 mol n NaOH = MV = [(1.0 mol/dm 3 ) (25 cm 3 )]÷1000 = 0.025 mol BACK](https://image.slidesharecdn.com/stoichiometry-111012004856-phpapp02/85/Stoichiometry-113-320.jpg)















































