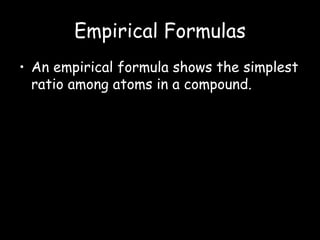
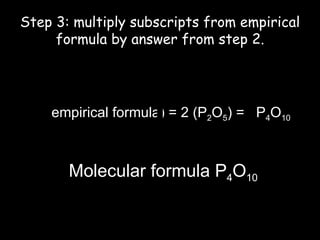
This document discusses empirical formulas and how to determine them from percentage composition or experimental data. It provides examples of calculating empirical formulas from the mass percentages of elements in a compound or the grams of elements in a sample. It also explains how to determine the molecular formula of a compound from the empirical formula and experimental molar mass. Key steps include converting percentages to grams of elements, calculating moles of each element, and dividing by the smallest ratio of elements to give whole number ratios in the empirical formula. The molecular formula is found by dividing the experimental molar mass by the molar mass of the empirical formula.