Embed presentation
Downloaded 124 times
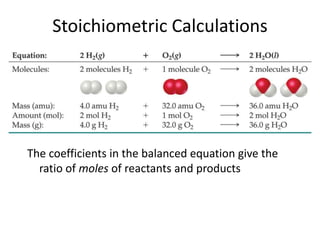

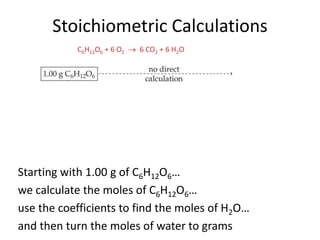


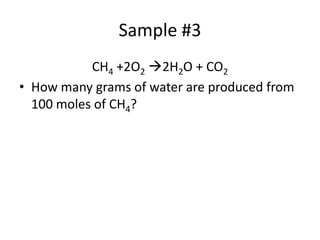



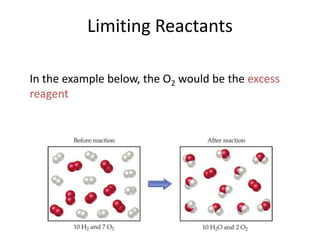
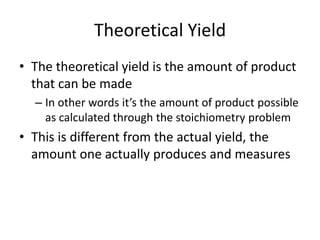
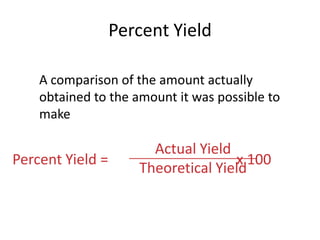
The document discusses stoichiometric calculations and limiting reactants. It explains that stoichiometric calculations use the mole ratios from balanced chemical equations to determine the amount of reactants and products. The limiting reactant is the one that limits the amount of product that can be formed. The theoretical yield is the maximum amount of product possible based on the stoichiometry, while the actual yield is the amount experimentally obtained. Percent yield compares the actual and theoretical yields.











