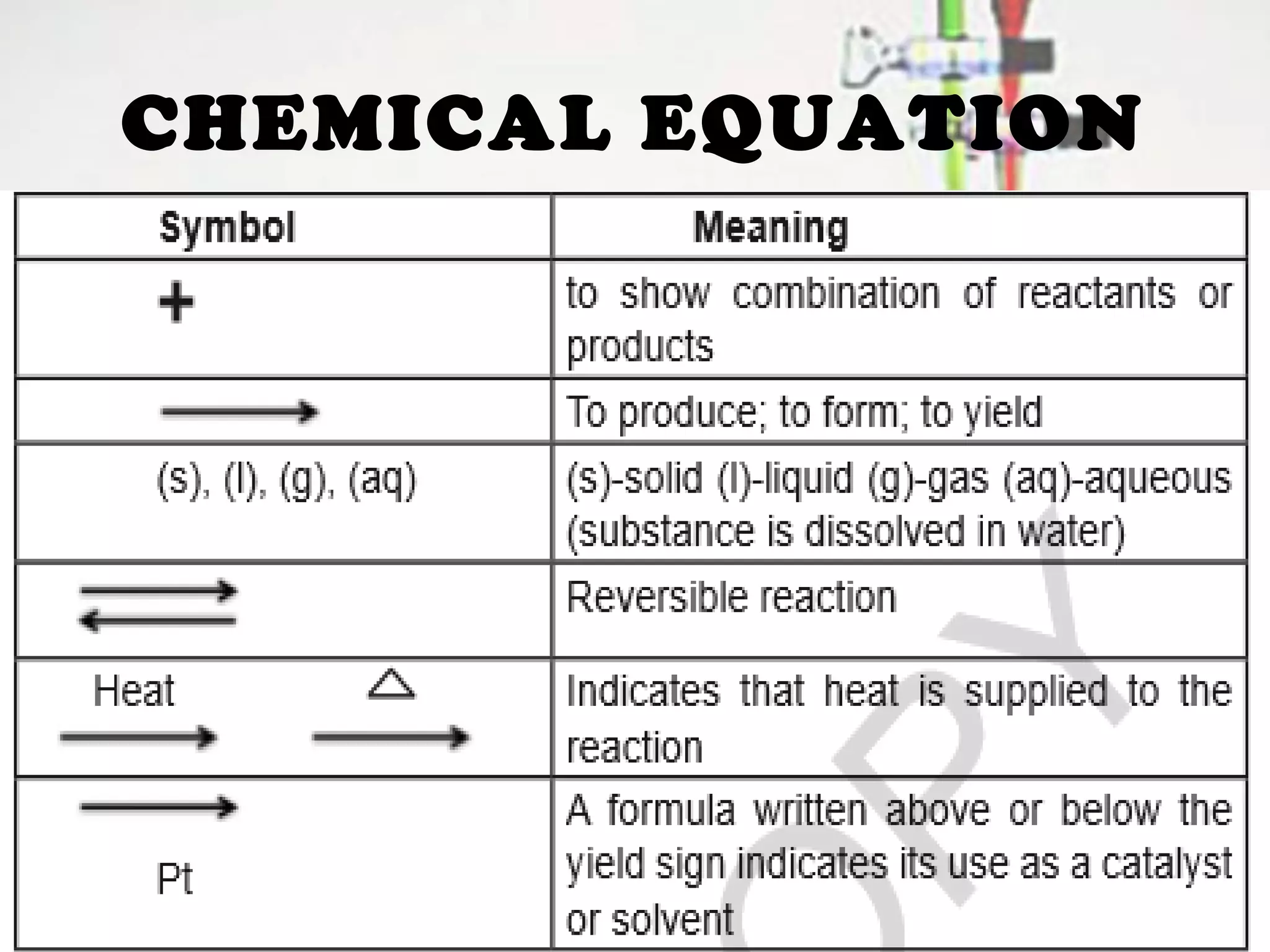
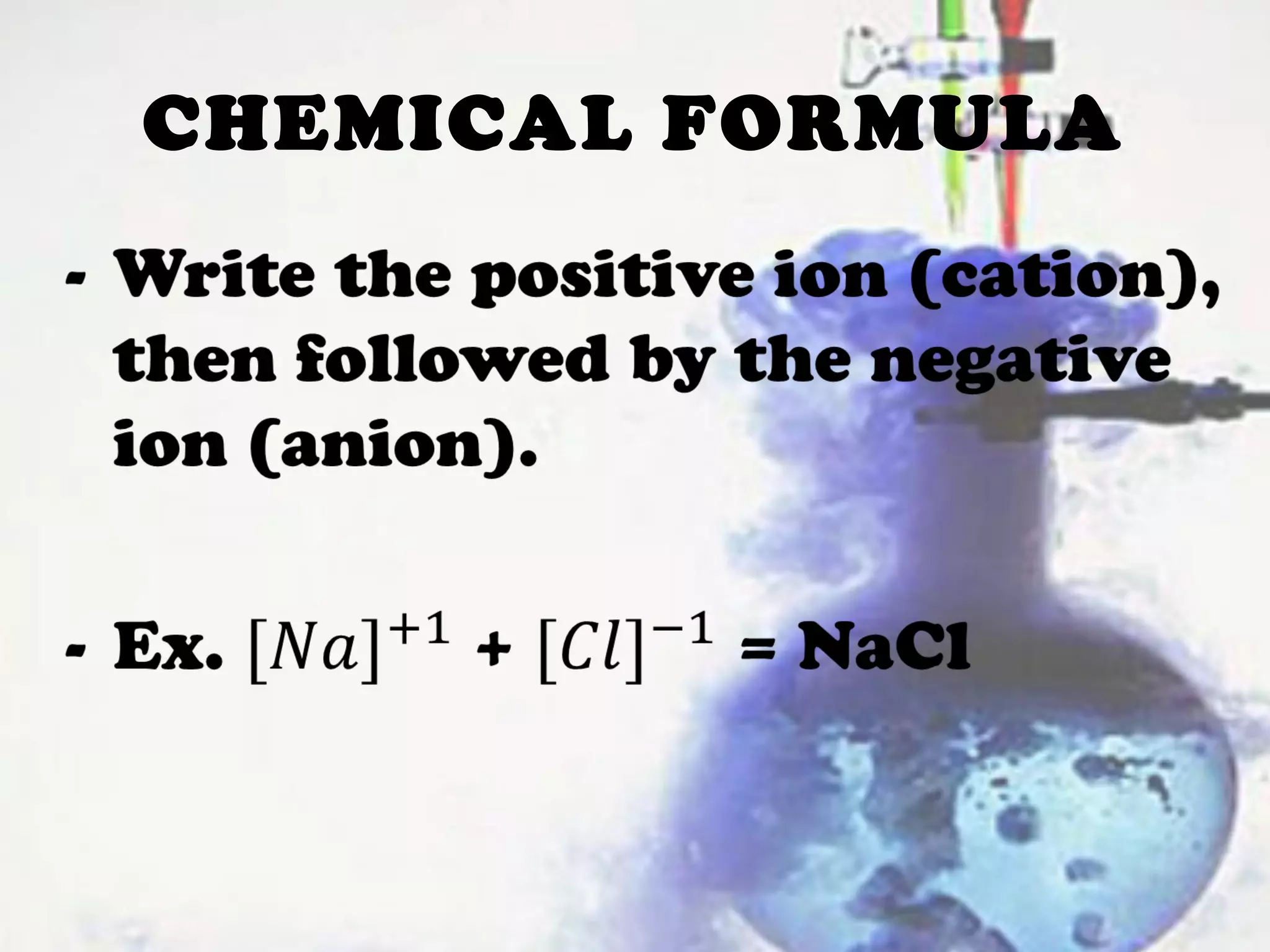
Chemical reactions involve the transformation of reactants into products, changing their physical and chemical properties. Key evidence of these reactions includes energy changes, color or odor changes, gas evolution, and precipitation formation. Various types of reactions, such as synthesis, decomposition, displacement, and combustion, are influenced by factors like temperature, particle size, catalysts, and concentration.